Trioxsalen
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
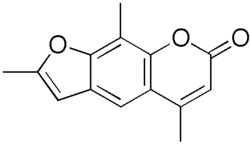
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Trioxsalen | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 12 O 3 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 228.24 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.22 g cm −3 |
||||||||||||||||||
| Melting point |
229-231 ° C |
||||||||||||||||||
| solubility |
soluble in chloroform (50 g l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Trioxsalen is a chemical compound from the group of furocoumarins and psoralen derivatives .
Occurrence
Trioxsalen is found in various plants, especially Psoralea corylifolia .
Extraction and presentation
Trioxsalen can be obtained by a multi-stage reaction starting from malonic acid with acetophenone , 2,3-dibromoethene and heating in a solvent such as N , N- diethylaniline . It is also possible to start from 2-methylresorcinol and its reaction with acetoacetate , allyl bromide , acetic anhydride and subsequent bromination and reaction with sodium methoxide .
properties
Trioxsalen is a light-sensitive, white solid that is soluble in chloroform .
use
Trioxsalen is used for the photochemical cross-linking of DNA as a probe for the nucleic acid structure and its function. Trioxsalen is pharmacologically inactive, but when exposed to UV radiation or sunlight it is converted to an active metabolite that can be used in the treatment of vitiligo , psoriasis and tumors . It is only approved for dermatological treatment in Scandinavia and the USA. The compound was launched in 1965.
Individual evidence
- ↑ a b c d e f g h i Data sheet trioxsalen, ≥98% (HPLC), powder from Sigma-Aldrich , accessed on March 18, 2016 ( PDF ).
- ^ Carl L. Yaws: The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics . Gulf Professional Publishing, 2015, ISBN 978-0-12-801146-1 , pp. 442 ( limited preview in Google Book search).
- ↑ a b Entry on trioxsalen in the DrugBank of the University of Alberta , accessed on June 29, 2019.
- ↑ Daniel Lednicer: Strategies for Organic Drug Synthesis and Design . John Wiley & Sons, 2009, ISBN 0-470-39959-7 , pp. 432 ( limited preview in Google Book search).
- ^ David B. Troy, Paul Beringer: Remington The Science and Practice of Pharmacy . Lippincott Williams & Wilkins, 2006, ISBN 978-0-7817-4673-1 , pp. 1293 ( limited preview in Google Book Search).
- ↑ M. Gloor, K. Thoma, J. Fluhr: Dermatological external therapy with special consideration of the magistral recipe . Springer-Verlag, 2013, ISBN 978-3-642-58308-7 , pp. 379 ( limited preview in Google Book search).
- ↑ Hans C. Korting: Dermatotherapy A Guide . Springer-Verlag, 2013, ISBN 978-3-642-79531-2 , pp. 168 ( limited preview in Google Book search).
- ^ Ronald I. Shorr: Drugs for the Geriatric Patient Text with BONUS Handheld Software . Elsevier Health Sciences, 2007, ISBN 978-1-4377-1035-9 , pp. 1278 ( limited preview in Google Book search).
- ^ William Andrew Publishing: Pharmaceutical Manufacturing Encyclopedia . Elsevier, 2013, ISBN 0-8155-1856-0 , pp. 3353 ( limited preview in Google Book search).

