Vinpocetine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
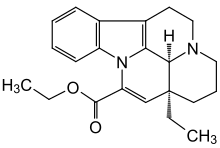
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Vinpocetine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 22 H 26 N 2 O 2 | ||||||||||||||||||
| Brief description |
white to pale yellow, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 350.46 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
149 to 152 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Vinpocetine is a semi-synthetic derivative of (+) - vincamine , the main alkaloid of the little evergreen , and is used as a medicinal substance.
Pharmacological properties
Vinpocetine causes the blood vessels in the brain to expand in vivo (cerebral vasodilation ) and increases the cerebral catecholamine metabolism. It inhibits u. a. the phosphodiesterase type 1. The exact mechanism of action is not known.
Clinical information
application areas
Cerebrovascular disorders, dementia and mild to moderate psychosyndrome are indicated as areas of application . The effectiveness of vinpocetine for use in Alzheimer's disease has been debated.
Side effects
Side effects reported include headaches, sleep disorders, gastrointestinal disorders, drop in blood pressure and an increased heart rate ( tachycardia ).
Other Information
Medicines containing vincopocetin (Cavinton ® ) have not been on the market in Germany since 2006, due to the expiry of the fictitious approval . Vinpocetine is monographed as a medicinal substance in the European Pharmacopoeia .
In addition to being a medicinal substance, vinpocetine is also used as a chiral reagent in stereoselective syntheses.
Individual evidence
- ↑ a b c d data sheet VINPOCETINE CRS (PDF) at EDQM , accessed on April 30, 2009.
- ↑ a b c d e f Commentary on the European Pharmacopoeia 6.0, loose-leaf collection, 30th edition 2008.
- ↑ a b data sheet Vinpocetine from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ^ Medina AE, Krahe TE, Ramoa AS. Restoration of neuronal plasticity by a phosphodiesterase type 1 inhibitor in a model of fetal alcohol exposure. J Neurosci. 2006; 26 : 1057-1060. PMID 16421325 .
- ↑ European Pharmacopoeia, 6th edition, basic work 2008, 6.0 / 2139.