Vitexin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
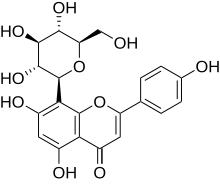
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Vitexin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 20 O 10 | ||||||||||||||||||
| Brief description |
yellow powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 432.38 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
260-263 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Vitexin is a naturally occurring chemical compound . As 8-C-glucosyl- apigenin , Vitexin is a derivative of apigenin , a plant pigment from the group of flavonoids . The C-glycoside is strictly speaking not a real glycoside , however, is often attributed to this group. Vitexin acts like many other flavonoids as antioxidants . In isolated form it is a crystalline yellow powder.
Occurrence

Blue passion flower ( Passiflora caerulea )
Vitexin occurs in the passion flower , in nettles and often in hawthorn leaves . It was also found in the leaves of some types of bamboo, as well as in knotweed ( Persicaria ), rye ( Secale cereale ) and in the seeds of fenugreek ( Trigonella foenum-graecum ).
Structurally related
One isomer of vitexin is isovitexin (apigenin-6-C-glucoside).
Individual evidence
- ↑ a b c Data sheet Vitexin at Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ SK Kapoor, PI Ahmad, A. Zaman: Chemical constituents of ailanthus excelsa . In: Phytochemistry . tape 10 , no. 12 , 1971, p. 3333 , doi : 10.1016 / S0031-9422 (00) 97424-7 .
- ↑ Entry on Vitexin in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Planta Medica . Vol. 43, 1981, p. 396.
- ↑ T. Dingermann, K. Hiller, G. Schneider, I. Zündorf: Schneider drug drugs. 5th edition. Elsevier 2004, ISBN 3-8274-1481-4 , p. 195.
- ↑ Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection . In: Food Chemistry . tape 143 , January 15, 2014, ISSN 0308-8146 , p. 48–53 , doi : 10.1016 / j.foodchem.2013.07.097 ( sciencedirect.com [accessed March 30, 2018]).
- ↑ T. Dingermann, K. Hiller, G. Schneider, I. Zündorf: Schneider drug drugs. 5th edition. Elsevier 2004, ISBN 3-8274-1481-4 , p. 199.
- ↑ Y. Zhang, J. Jiao, C. Liu, X. Wu, Zhang, Y: Isolation and purification of four flavone C-glycosides from antioxidant of bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography. In: Food Chemistry . 2007. doi: 10.1016 / j.foodchem.2007.09.037 .
- ^ R. Hänsel , K. Keller, H. Rimpler, G. Schneider (Eds.): Hager's Handbook of Pharmaceutical Practice. Volume 6: Drugs P-Z. 5th edition. Springer Berlin / Heidelberg / New York 1994, ISBN 3-540-52639-0 , p. 74.
- ^ R. Hänsel, K. Keller, H. Rimpler, G. Schneider (Eds.): Hager's Handbook of Pharmaceutical Practice. Volume 6: Drugs P-Z. 5th edition. Springer Berlin / Heidelberg / New York 1994, ISBN 3-540-52639-0 , p. 649.
- ^ R. Hänsel, K. Keller, H. Rimpler, G. Schneider (Eds.): Hager's Handbook of Pharmaceutical Practice. Volume 6: Drugs P-Z. 5th edition. Springer Berlin / Heidelberg / New York 1994, ISBN 3-540-52639-0 , p. 1000.