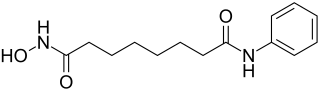
Vorinostat
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Vorinostat | |||||||||||||||||||||
| other names |
N -hydroxy- N ′ -phenyloctanediamide ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 14 H 20 N 2 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Cytostatic |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 264.32 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
159-160.5 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Vorinostat (trade name in the USA: Zolinza) is an aromatic chemical compound from the group of hydroxamic acids , used as a drug to be administered orally for histone deacetylase inhibition . It is approved for the treatment of advanced, refractory, cutaneous T-cell lymphoma . Other current clinical studies concern the effects of vorinostat in HIV , colon cancer and other cancers, as well as in combination therapies in solid tumors.
pharmacology
Histones represent the protein component of chromatin . Acetylation and deacetylation of the histones regulate the transcription of the DNA. An inhibition of the histone deacetylase leads to a hyperacetylation of the histones. This can u. a. lead to controlled cell death ( apoptosis ) of the cancer cells .
literature
- W. Xu, L. Ngo, G. Perez, M. Dokmanovic, PA Marks: Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitor. In: Proceedings of the National Academy of Sciences . Volume 103, number 42, October 2006, pp. 15540-15545, doi : 10.1073 / pnas.0607518103 , PMID 17030815 , PMC 1592530 (free full text).
- LK Gediya, P. Chopra, P. Purushottamachar, N. Maheshwari, VC Njar: A new simple and high-yield synthesis of suberoylanilide hydroxamic acid and its inhibitory effect alone or in combination with retinoids on proliferation of human prostate cancer cells. In: Journal of medicinal chemistry. Volume 48, Number 15, July 2005, pp. 5047-5051, doi : 10.1021 / jm058214k , PMID 16033284 .
Web links
- With cancer drug against HIV - AIDS experts hope for a cure. At: ntv.de on March 19, 2012
Individual evidence
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 1728, ISBN 978-0-911910-00-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of N-Hydroxy-N-phenyloctanediamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 13, 2020, is reproduced from a self-classification by the distributor .
