Karl Kässbohrer Fahrzeugwerke and User:Marshallsumter: Difference between pages
m tidy up |
|||
| Line 1: | Line 1: | ||
[[Biosynthesis]] of a [[Human]] [[Protein]] |
|||
'''Kässbohrer Fahrzeugwerke''' was a German vehicle manufacturer in [[Ulm]]. Its products were [[bus]]es, [[Coach (vehicle)|coaches]], [[vehicle transporter]]s, [[trailer]]s and special vehicles like [[Snow grooming|snow groomer vehicles]]. |
|||
{{Taxobox |
|||
In 1893 Karl Kässbohrer founded the ''Wagenfabrik Kässbohrer'' in Ulm. In 1922 Kässbohrer developped a trailer for goods transport, having solid rubber wheels. When Karl Kässbohrer senior died, his sons, Karl junior und Otto Kässbohrer took over the company. In 1969, Kässbohrer was Germany's biggest coach and truck trailer producer. Kässbohrer's coaches and buses were named [[Setra]]. The snow grooming vehicles were called ''PistenBully'' and 2,000 were sold between 1979 and 1989. At the end of the 1990s about 9,000 employees worked for Kässbohrer. |
|||
| color |
|||
| name = [[Human]] Lineage |
|||
| domain = [[Eukaryote|Eukaryota]] |
|||
| regnum = [[Animal]]ia|Metazoa]] |
|||
| phylum = [[Chordate|Chordata]] |
|||
| subdivisio = [[Craniata]] |
|||
| subphylum = [[Vertebrate|Vertebrata]] |
|||
| infraphylum = [[Euteleostomi]] |
|||
| classis = [[Mammal|Mammalia]] |
|||
| infraclassis = [[Eutheria]] |
|||
| superordo = [[Euarchontoglires]] |
|||
| ordo = [[Primate|Primates]] |
|||
| subordo = [[Haplorrhini]] |
|||
| infraordo = [[Simiiformes]] |
|||
| parvordo = [[Catarrhini]] |
|||
| parvordo_authority = [[Étienne Geoffroy Saint-Hilaire|É. Geoffroy]], 1812 |
|||
| familia = [[Great ape|Hominidae]] |
|||
| subfamilia = [[Homininae]] |
|||
| tribus = [[Hominini]] |
|||
| subtribus = [[Hominina]] |
|||
| genus = [[Homo (genus)|Homo]] |
|||
| genus_authority = [[Carolus Linnaeus|Linnaeus]], 1758 |
|||
| species = sapiens |
|||
| subspecies = sapiens |
|||
''[[Human|Homo sapiens]] sapiens''<br> |
|||
}} |
|||
Consider starting with a specific gene. Assemble the transcription mechanism at the appropriate genome location. Transcribe the gene using nucleotide accountability. |
|||
Starting in 1993, the company began to fall apart. The division's truck bodies, semi-trailers and trailers were sold to the competitor [[Kögel]]. In 1994 the snow groomer vehicles division was spun off to Kässbohrer Geländefahrzeug (all-terrain vehicle) GmbH. In the end, Mercedes-Benz bought the bus and coach division in 1995 and the new name [[EvoBus]] was introduced. The only division which is still family owned, is ''Kässbohrer Transport Technik'' in [[Salzburg]], [[Austria]], where vehicle transporters are built. |
|||
=[[Protein biosynthesis]]= |
|||
A remarkable number of Kässbohrer Setra coaches and buses are still in service on German roads, sometimes having a mileage of more than a million kilometers. |
|||
Alpha-amino acids are the building blocks of [[protein]]s. Amino acids combine in a [[condensation reaction]] (through dehydration synthesis) that releases water and the new "amino acid residue" held together by a [[peptide bond]]. Proteins are defined by their unique sequence of amino acid residues; this sequence is the [[primary structure]] of the protein. |
|||
===Gallery=== |
|||
<center><gallery> |
|||
Image:Setra-Bus mit Panoramafenstern.jpg|Kässbohrer Setra S9 coach, built between 1959 and 1967 |
|||
Image:Setra 215HD 1981.jpg|Kässbohrer Setra S215HD (1976-1991) coach |
|||
Image:Kässbohrer PistenBully PB 280 D.jpg|Kässbohrer snow groomer vehicle |
|||
</gallery></center> |
|||
=[[Amino acid synthesis]]= |
|||
[[Biochemical]] processes ([[metabolic pathways]]) produce [[amino acid]]s from other [[compounds]]. The substrates for these processes are various compounds in a [[Human]]'s diet or are produced resident flora in the gut. Humans are only able to synthesise 12 of the 20 standard amino acids. |
|||
== External links == |
|||
* [http://www.setra.de/ Website Setra] |
|||
* [http://www.pistenbully.com/ Website Kässbohrer all-terrain vehicle, division Pistenbully] |
|||
* [http://www.beach-tech.com/ Website Kässbohrer all-terrain vehicle, division BeachTech] |
|||
* [http://www.kaessbohrer.at/index.php?id=23&L=1 Kässbohrer vehicle transporters (English language website)] |
|||
Amino acids are synthesized from [[Glutamate]], which is formed by [[amination]] of α-ketoglutarate: |
|||
<math>\alpha -ketoglutarate + NH_4^+ \rightleftarrows Glutamate</math> |
|||
[[Category:Companies of Germany]] |
|||
[[Category:Defunct motor vehicle manufacturers of Germany]] |
|||
Afterwards, [[Alanine]] and [[Aspartate]] are formed by [[transamination]] of [[Glutamate]]. |
|||
[[Category:Defunct bus manufacturers]] |
|||
All of the remaining amino acids are then constructed from [[Glutamate]] or [[Aspartate]], by [[transamination]] of these two amino acids with one α-keto acid. |
|||
[[Category:Motor vehicle manufacturers of Germany]] |
|||
[[Category:Bus manufacturers]] |
|||
== Synthesis from Intermediates of the Citric Acid Cycle and Other Major Pathways == |
|||
Human beings can synthesize 11 (nonessential) of the basic set of 20 amino acids. The essential amino acids, which must be supplied in the diet. The pathways for the synthesis of nonessential amino acids are quite simple. Glutamate dehydrogenase catalyzes the reductive amination of α-ketoglutarate to glutamate. A transamination reaction takes place in the synthesis of most amino acids. At this step, the chirality of the amino acid is established. [[Alanine]] and [[aspartate]] are synthesized by the transamination of [[pyruvate]] and [[oxaloacetate]], respectively. Glutamine is synthesized from NH4+ and glutamate, and [[asparagine]] is synthesized similarly. [[Proline]] and [[arginine]] are derived from glutamate. [[Serine]], formed from 3-phosphoglycerate, is the precursor of [[glycine]] and [[cysteine]]. [[Tyrosine]] is synthesized by the hydroxylation of [[phenylalanine]], an essential amino acid. The pathways for the biosynthesis of essential amino acids are much more complex than those for the nonessential ones. |
|||
[[Tetrahydrofolate]], a carrier of activated one-carbon units, plays an important role in the metabolism of amino acids and nucleotides. This [[coenzyme]] carries one-carbon units at three oxidation states, which are interconvertible: most reduced—methyl; intermediate—methylene; and most oxidized—formyl, formimino, and methenyl. The major donor of activated methyl groups is S-adenosylmethionine, which is synthesized by the transfer of an adenosyl group from ATP to the sulfur atom of methionine. S-Adenosylhomocysteine is formed when the activated methyl group is transferred to an acceptor. It is hydrolyzed to adenosine and homocysteine, the latter of which is then methylated to methionine to complete the activated methyl cycle. |
|||
==Nutritional importance== |
|||
{{further|[[Protein in nutrition]]}} |
|||
Of the 20 standard proteinogenic amino acids, 8 are called [[essential amino acid]]s because the [[human body]] cannot [[synthesize]] them from other [[chemical compound|compounds]] at the level needed for normal growth, so they must be obtained from food.<ref>{{cite journal |author=Young VR |title=Adult amino acid requirements: The case for a major revision in current recommendations |journal=J. Nutr. |volume=124 |issue=8 Suppl |pages=1517S–1523S |year=1994 |pmid=8064412 |url=http://jn.nutrition.org/cgi/reprint/124/8_Suppl/1517S.pdf}}</ref> However, the situation is a little more complicated since [[cysteine]], [[tyrosine]], [[histidine]] and [[arginine]] are semiessential amino acids in children, because the metabolic pathways that synthesize these amino acids are not fully developed.<ref>{{cite journal |author=Imura K, Okada A |title=Amino acid metabolism in pediatric patients |journal=Nutrition |volume=14 |issue=1 |pages=143–8 |year=1998 |pmid=9437700 |url=http://jn.nutrition.org/cgi/content/full/130/7/1835S |doi=10.1016/S0899-9007(97)00230-X}}</ref> The amounts required also depend on the age and health of the individual, so it is hard to make general statements about the dietary requirement for some amino acids. |
|||
{| class="wikitable" |
|||
! Essential |
|||
! Nonessential |
|||
|- |
|||
| [[Isoleucine]] |
|||
| [[Alanine]] |
|||
|- |
|||
| [[Leucine]] |
|||
| [[Asparagine]] |
|||
|- |
|||
| [[Lysine]] |
|||
| [[Aspartate]] |
|||
|- |
|||
| [[Methionine]] |
|||
| [[Cysteine]]* |
|||
|- |
|||
| [[Phenylalanine]] |
|||
| [[Glutamate]] |
|||
|- |
|||
| [[Threonine]] |
|||
| [[Glutamine]]* |
|||
|- |
|||
| [[Tryptophan]] |
|||
| [[Glycine]]* |
|||
|- |
|||
| [[Valine]] |
|||
| [[Proline]]* |
|||
|- |
|||
| |
|||
| [[Serine]]* |
|||
|- |
|||
| |
|||
| [[Tyrosine]]* |
|||
|- |
|||
| |
|||
| [[Arginine]]* |
|||
|- |
|||
| |
|||
| [[Histidine]]* |
|||
|} |
|||
(*) Essential only in certain cases.<ref>{{cite journal |author=Fürst P, Stehle P |title=What are the essential elements needed for the determination of amino acid requirements in humans? |journal=J. Nutr. |volume=134 |issue=6 Suppl |pages=1558S–1565S |year=2004 |pmid=15173430 |url=http://jn.nutrition.org/cgi/content/full/134/6/1558S}}</ref><ref>{{cite journal |author=Reeds PJ |title=Dispensable and indispensable amino acids for humans |journal=J. Nutr. |volume=130 |issue=7 |pages=1835S–40S |year=2000 |pmid=10867060 |url=http://jn.nutrition.org/cgi/content/full/130/7/1835S}}</ref> |
|||
==Regulation of Biosynthesis by Feedback Inhibition== |
|||
Most of the pathways of amino acid biosynthesis are regulated by [[feedback inhibition]], in which the committed step is allosterically inhibited by the final product. Branched pathways require extensive interaction among the branches that includes both negative and positive regulation. The regulation of glutamine synthetase from [[E. coli]] is a striking demonstration of cumulative feedback inhibition and of control by a cascade of reversible covalent modifications. |
|||
=[[Transcription (genetics)|Transcription]]= |
|||
A [[DNA]] sequence is [[Enzyme|enzymatically]] copied by [[RNA polymerase II]] to produce a complementary nucleotide [[RNA]] strand of messenger RNA (mRNA). mRNA is modified through [[RNA splicing]], 5' end capping, and the addition of a polyA tail.<ref> Robert J. Brooker ''Genetics: analysis and principles.'' 2nd edition. (New York: McGraw-Hill 2005) Chapter 12 "Gene transcription and RNA modification" pp. 318-325.</ref> |
|||
===[[Transcriber]]=== |
|||
In human [[Biochemistry|biochemistry]] a transcriber might be thought of as a tool assembled in the nucleus for the transcription of genetic information. It synthesizes (a nucleic acid, usually RNA) using an existing nucleic acid (DNA) as a template from which to copy. |
|||
===[[Transcription factor|Transcription factors]]=== |
|||
[[Transcription factor|Transcription factors]] bind to specific [[DNA sequence|sequences]] of [[DNA]] and are part of the system that controls the [[transcription (genetics)|transcription]] of genetic information from DNA to [[RNA]].<ref name="pmid9570129">{{cite journal | author = Latchman DS | title = Transcription factors: an overview | journal = Int. J. Biochem. Cell Biol. | volume = 29 | issue = 12 | pages = 1305–12 | year = 1997 | pmid = 9570129 | doi = 10.1016/S1357-2725(97)00085-X }}</ref><ref name="pmid2128034">{{cite journal | author = Karin M | title = Too many transcription factors: positive and negative interactions | journal = New Biol. | volume = 2 | issue = 2 | pages = 126–31 | year = 1990 | pmid = 2128034 | doi = | issn = }}</ref> Transcription factors occur in a complex, increase (as an [[Activator (genetics)|activator]]), or prevent (as a [[repressor]]) the presence of [[RNA polymerase II]].<ref name="pmid8870495">{{cite journal | author = Roeder RG | title = The role of general initiation factors in transcription by RNA polymerase II | journal = Trends Biochem. Sci. | volume = 21 | issue = 9 | pages = 327–35 | year = 1996 | pmid = 8870495 | doi = 10.1016/0968-0004(96)10050-5 }}</ref><ref name="pmid8990153">{{cite journal | author = Nikolov DB, Burley SK | title = RNA polymerase II transcription initiation: a structural view | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 94 | issue = 1 | pages = 15–22 | year = 1997 | pmid = 8990153 | doi = 10.1073/pnas.94.1.15 }}</ref><ref name="pmid11092823">{{cite journal | author = Lee TI, Young RA | title = Transcription of eukaryotic protein-coding genes | journal = Annu. Rev. Genet. | volume = 34 | issue = | pages = 77–137 | year = 2000 | pmid = 11092823 | doi = 10.1146/annurev.genet.34.1.77}}</ref> |
|||
Each transcription factor contains one or more [[DNA binding domain]]s (DBDs) which attach to specific sequences of DNA adjacent to the gene that it regulates.<ref name="pmid2667136">{{cite journal | author = Mitchell PJ, Tjian R | title = Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins | journal = Science | volume = 245 | issue = 4916 | pages = 371-8 | year = 1989 | pmid = 2667136 | doi = 10.1126/science.2667136 | issn = }}</ref><ref name="pmid9121580">{{cite journal | author = Ptashne M, Gann A | title = Transcriptional activation by recruitment | journal = Nature | volume = 386 | issue = 6625 | pages = 569-77 | year = 1997 | pmid = 9121580 | doi = 10.1038/386569a0 | issn = }}</ref> |
|||
There are approximately 2600 proteins in the [[human genome]] that contain DNA-binding domains and most of these are presumed to function as transcription factors.<ref name="pmid15193307">{{cite journal | author = Babu MM, Luscombe NM, Aravind L, Gerstein M, Teichmann SA | title = Structure and evolution of transcriptional regulatory networks | journal = Curr. Opin. Struct. Biol. | volume = 14 | issue = 3 | pages = 283–91 | year = 2004 | pmid = 15193307 | doi = 10.1016/j.sbi.2004.05.004 }}</ref> |
|||
The combinatorial use of a subset of the approximately 2000 human transcription factors easily accounts for the unique regulation of each gene in the human genome during [[developmental biology|development]].<ref name="pmid11823631" /> |
|||
[[General transcription factor]]s (GTFs) are necessary for transcription to occur. GTFs are part of the large transcription [[preinitiation complex]] that interacts with [[RNA polymerase II]] directly.<ref name="pmid16858867">{{cite journal | author = Thomas MC, Chiang CM | title = The general transcription machinery and general cofactors | journal = Critical reviews in biochemistry and molecular biology | volume = 41 | issue = 3 | pages = 105–78 | year = 2006 | pmid = 16858867 | doi = | url = | issn = }}</ref> They are ubiquitous and interact with the core promoter region surrounding the transcription start site(s) of all[[class II gene]]s.<ref name="pmidc">{{cite journal | author = Orphanides G, Lagrange T, Reinberg D | title = The general transcription factors of RNA polymerase II | journal = Genes Dev. | volume = 10 | issue = 21 |pages = 2657–83 | year = 1996 | pmid = 8946909 | doi = 10.1101/gad.10.21.2657 }}</ref> |
|||
[[TFIIA]] |
|||
[[TFIIB]] |
|||
[[TFIID]] (see also [[TATA binding protein]]) |
|||
[[TFIIE]] |
|||
[[TFIIF]] |
|||
[[TFIIH]] |
|||
Active transcription units (transcription factors) are clustered in the nucleus. There are ~10,000 factors in the nucleoplasm of a [[HeLa cell]], among which are ~8,000 polymerase II factors and ~2,000 polymerase III factors. Each polymerase II factor contains ~8 polymerases. As most active transcription units are associated with only one polymerase, each factor will be associated with ~8 different transcription units. These units might be associated through promoters and/or enhancers, with loops forming a ‘cloud’ around the factor. |
|||
Additional proteins: |
|||
[[coactivator (genetics)|coactivator]]s |
|||
[[Chromatin Structure Remodeling (RSC) Complex|chromatin remodeler]]s |
|||
[[histone acetyltransferase|histone acetylases]] |
|||
[[histone deacetylase| deacetylases]] |
|||
[[kinase]]s |
|||
[[methylase]]s |
|||
play crucial roles in [[regulation of gene expression|gene regulation]].<ref name="pmid11823631">{{cite journal | author = Brivanlou AH, Darnell JE | title = Signal transduction and the control of gene expression | journal = Science | volume = 295 | issue = 5556 | pages = 813-8 | year = 2002 | pmid = 11823631 | doi = 10.1126/science.1066355 | issn = }}</ref> |
|||
===[[RNA polymerase II]]=== |
|||
Each of the [[RNA polymerase|RNA polymerases]] transcribes different classes of proteins. Specifically, [[RNA polymerase I]] transcribes [[rRNA]] and [[RNA polymerase III]] transcribes various additional RNAs. |
|||
[[RNA polymerase II]] transcribes [[Class II gene|Class II genes]] to form proteins. It catalyzes the [[Transcription (genetics)|transcription]] of [[DNA]] to synthesize precursors of [[mRNA]] and most [[snRNA]] and [[microRNA]].<ref>{{cite journal | author = Kronberg, R.D. | title = Eukaryotic transcriptional control | journal = Trends Cell Biol. | year=1999 | volume=9 | issue=12 | pages=M46–49 | pmid = 10611681 | doi = 10.1016/S0962-8924(99)01679-7}}</ref> |
|||
It is a 550 [[kDa]] complex of 12 subunits. |
|||
{{PBB|geneid=5430}} |
|||
'''Polymerase (RNA) II (DNA directed) polypeptide A, 220kDa''', also known as '''POLR2A''', is a human [[gene]]. |
|||
<!-- The PBB_Summary template is automatically maintained by Protein Box Bot. See Template:PBB_Controls to Stop updates. --> |
|||
{{PBB_Summary |
|||
| section_title = |
|||
| summary_text = This gene encodes the largest subunit of RNA polymerase II, the polymerase responsible for synthesizing messenger RNA in eukaryotes. The product of this gene contains a carboxy terminal domain composed of heptapeptide repeats that are essential for polymerase activity. These repeats contain serine and threonine residues that are phosphorylated in actively transcribing RNA polymerase. In addition, this subunit, in combination with several other polymerase subunits, forms the DNA binding domain of the polymerase, a groove in which the DNA template is transcribed into RNA.<ref>{{cite web | title = Entrez Gene: POLR2A polymerase (RNA) II (DNA directed) polypeptide A, 220kDa| url = http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5430| accessdate = }}</ref> |
|||
}} |
|||
{{PBB|geneid=5431}} |
|||
'''Polymerase (RNA) II (DNA directed) polypeptide B, 140kDa''', also known as '''POLR2B''', is a human [[gene]]. |
|||
<!-- The PBB_Summary template is automatically maintained by Protein Box Bot. See Template:PBB_Controls to Stop updates. --> |
|||
{{PBB_Summary |
|||
| section_title = |
|||
| summary_text = This gene encodes the second largest subunit of RNA polymerase II, the polymerase responsible for synthesizing messenger RNA in eukaryotes. This subunit, in combination with at least two other polymerase subunits, forms a structure within the polymerase that maintains contact in the active site of the enzyme between the DNA template and the newly synthesized RNA.<ref>{{cite web | title = Entrez Gene: POLR2B polymerase (RNA) II (DNA directed) polypeptide B, 140kDa| url = http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5431| accessdate = }}</ref> |
|||
}} |
|||
{{PBB|geneid=6908}} |
|||
The '''TATA binding protein''' ('''TBP''') is a [[transcription factor]] that binds specifically to a [[DNA]] sequence called the [[TATA box]]. This DNA sequence is found about 25-30 [[base pair]]s upstream of the transcription start site in some [[eukaryote|eukaryotic]] [[gene]] [[promoter]]s.<ref name="pmid17670940">{{cite journal | author = Kornberg RD | title = The molecular basis of eukaryotic transcription | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 104 | issue = 32 | pages = 12955–61 | year = 2007 | pmid = 17670940 | doi = 10.1073/pnas.0704138104}}</ref> TBP, along with a variety of [[TBP-associated factor]]s, make up the [[TFIID]], a [[general transcription factor]] that in turn makes up part of the [[RNA polymerase II]] [[preinitiation complex]].<ref name="pmid11092823">{{cite journal | author = Lee TI, Young RA | title = Transcription of eukaryotic protein-coding genes | journal = Annu. Rev. Genet. | volume = 34 | issue = | pages = 77–137 | year = 2000 | pmid = 11092823 | doi = 10.1146/annurev.genet.34.1.77}}</ref> As one of the few proteins in the preinitation complex that binds DNA in a sequence-specific manner, it helps position RNA polymerase II over the transcription start site of the gene. However, it is estimated that only 10-20% of human promoters have TATA boxes. Therefore, TBP is probably not the only protein involved in positioning RNA polymerase II. |
|||
TBP is a subunit of the eukaryotic [[transcription factor]] TFIID. [[TFIID]] is the first protein to bind to DNA during the formation of the pre-initiation [[Transcription (genetics)|transcription]] complex of [[RNA polymerase II]] (RNA Pol II). Binding of TFIID to the [[TATA box]] in the [[promoter]] region of the gene initiates the recruitment of other factors required for RNA Pol II to begin transcription. Some of the other recruited [[transcription factors]] include [[TFIIA]], [[TFIIB]] and [[TFIIF]]. Each of these transcription factors are formed from the interaction of many protein subunits, indicating that transcription is a heavily regulated process. |
|||
When TBP binds to a [[TATA box]] within the [[DNA]], it distorts the DNA by inserting amino acid side chains between base pairs, partially unwinding the helix, and doubly kinking it. The distortion is accomplished through a great amount of surface contact between the protein and DNA. TBP binds with the negatively charged phosphates in the DNA backbone through positively charged [[lysine]] and [[arginine]] amino acid residues. The sharp bend in the DNA is produced through projection of four bulky [[phenylalanine]] residues into the minor groove. As the DNA bends, its contact with TBP increases, thus enhancing the DNA-protein interaction. |
|||
The strain imposed on the DNA through this interaction initiates melting, or separation, of the strands. Because this region of DNA is rich in [[adenine]] and [[thymine]] residues, which base pair through only two [[hydrogen bonds]], the DNA strands are more easily separated. Separation of the two strands exposes the bases and allows [[RNA polymerase II]] to begin transcription of the [[gene]]. |
|||
====Initiation Regulation==== |
|||
Initiation is regulated by several mechanisms: |
|||
#Protein interference. |
|||
#[[Chromatin]] structure inhibition. |
|||
#Phosphorylation. |
|||
=====Regulation by Protein interference===== |
|||
Protein interference is the process where some signaling protein interacts, either with the promoter or some stage of the partially constructed complex, to prevent further construction of the polymerase complex. This is generally a very rapid response and is used for fine level, individual gene control and for 'cascade' processes for a group of genes useful under a specific conditions (for example DNA repair genes or heat shock genes). |
|||
=====Regulation by Chromatin structure inhibition===== |
|||
[[Chromatin]] structure inhibition is the process where the promoter is hidden by [[chromatin]] structure. Chromatin structure is controlled by post-translational modification of the [[histone]]s involved and leads to gross levels of high or low transcription levels. See: [[chromatin]], [[histone]] and [[nucleosome]]. |
|||
These methods of control can be combined in a modular method, allowing very high specificity in transcription initiation control. |
|||
=====Regulation by Phosphorylation===== |
|||
The largest subunit of Pol II (Rpb1) has a domain at its C-terminus that is called the CTD (C-terminal domain). This is the target of [[protein kinase|kinases]] and [[phosphatases]]. The phosphorylation of the CTD is an important regulation mechanism, as this allows attraction and rejection of factors that have a function in the transcription process. The CTD can be considered as a platform for [[transcription factors]]. |
|||
The CTD consists of repetitions of an [[amino acid]] motif, YSPTSPS, of which [[Serine]]s and [[Threonine]]s can be [[phosphorylated]]. The mammalian protein contains 52. There are many different combinations of phosphorylations possible on these repeats and these can change rapidly during transcription. The regulation of these phosphorylations and the consequences for the association of transcription factors plays a major role in the regulation of transcription. |
|||
During the transcription cycle, the CTD of the large subunit of RNAP II is reversibly phosphorylated. RNAP II containing unphosphorylated CTD is recruited to the promoter, whereas the hyperphosphorylated CTD form is involved in active transcription. Phosphorylation occurs at two sites within the heptapeptide repeat, at Serine 5 and Serine 2. Serine 5 phosphorylation is confined to promoter regions and is necessary for the initiation of transcription, whereas Serine 2 phosphorylation is important for mRNA elongation and 3'-end processing. |
|||
===[[Mediator (coactivator)]]=== |
|||
==Messenger RNA== |
|||
[[Image:MRNA-interaction.png|thumb|300px| |
|||
The "life cycle" of an '''mRNA''' in a eukaryotic cell. RNA is [[transcription (genetics)|transcribed]] in the [[cell nucleus|nucleus]]; once completely processed, it is transported to the [[cytoplasm]] and [[Translation (genetics)|translated]] by the [[ribosome]]. At the end of its life, the mRNA is degraded.]] |
|||
In the ribosome, the nucleic acid polymer is [[translation (genetics)|translated]] into a polymer of [[amino acids]]: a protein. |
|||
[[Image:Transcription label fromcommons.jpg|thumb|250px|right|A micrograph of ongoing gene transcription of [[ribosomal RNA]] illustrating the growing [[primary transcript]]s. "Begin" indicates the [[3' end]] of the DNA template strand, where new RNA synthesis begins; "end" indicates the [[5' end]], where the primary transcripts are almost complete.]] |
|||
==Pre-Initiation== |
|||
RNA polymerase II binds to the DNA and, along with other cofactors, unwinds the DNA to create an initiation bubble so that the polymerase has access to the single-stranded DNA template. RNA Polymerase II does require a promoter like sequence. |
|||
Proximal (core) Promoters: |
|||
TATA promoters are found around -30 bp to the start site of transcription. Not all genes have TATA box promoters and there exists TATA-less promoters as well. The TATA promoter consensus sequence is TATA(A/T)A(A/T). |
|||
The following are the steps involved in TATA Promoter Complex formation: |
|||
1. General transcription factors bind |
|||
2. TFIID, TFIIA, TFIIB, TFIIF (w/RNA Polymerase II), TFIIH/E |
|||
From the beginning of complex formation, the pre-initiation complex is closed. |
|||
==Initiation== |
|||
[[Image:simple transcription initiation1.svg|thumb|400px|Simple diagram of transcription initiation. RNAP = RNA polymerase II]] |
|||
A collection of [[Transcription factor|transcription factors]] mediate the binding of RNA polymerase II and the initiation of transcription. Only after certain transcription factors are attached to the promoter does the polymerase bind to it. The completed assembly of transcription factors and RNA polymerase II (transcription initiation complex) bind to the promoter. |
|||
Once the complex is opened by TFIIH initiation starts and the first phosphodiester bond is formed. |
|||
==Promoter Clearance== |
|||
During the formation of the first nucleotide bond the RNA polymerase begins to clear the promoter. There is a tendency to release the RNA transcript and produce truncated transcripts (abortive initiation). Once the transcript reaches approximately 23 nucleotides it no longer slips and Elongation can occur. This is an ATP dependent process. The short-lived, unprocessed or partially processed, product is termed ''[[pre-mRNA]]''. |
|||
Promoter clearance also coincides with Phosphorylation of serine 5 on the carboxy terminal domain which is phosphorylated by TFIIH. |
|||
== Eukaryotic pre-mRNA processing == |
|||
{{main|Post-transcriptional modification}} |
|||
Pre-mRNA requires extensive processing. |
|||
==== 5' cap addition ==== |
|||
{{main|5' cap}} |
|||
A ''5' cap'' (also termed an RNA cap, an RNA 7-methylguanosine cap or an RNA m<sup>7</sup>G cap) is a modified guanine nucleotide that is added to the "front" or [[5' end]] of a the pre-mRNA using a 5',5-Triphosphate linkage shortly after the start of transcription. The 5' cap consists of a terminal 7-methylguanosine residue which is linked through a 5'-5'-triphosphate bond to the first transcribed nucleotide. Its presence is critical for recognition and proper attachment of mRNA by the [[ribosome]] and protection from 5' exonucleases [[RNase]]s. |
|||
Cap addition is coupled to transcription, and occurs co-transcriptionally, such that each influences the other. Shortly after the start of transcription, the 5' end of the mRNA being synthesized is bound by a cap-synthesizing complex associated with [[RNA polymerase]]. This [[enzyme|enzymatic]] complex [[catalyze]]s the chemical reactions that are required for mRNA capping. Synthesis proceeds as a multi-step [[biochemistry|biochemical]] reaction. |
|||
=== Coding regions === |
|||
{{main|Coding region}} |
|||
Coding regions are composed of [[codons]], which are decoded and translated into one protein by the ribosome. Coding regions begin with the [[start codon]] and end with the a [[stop codon| stop codons]]. Generally, the start codon is an AUG triplet and the stop codon is UAA, UAG, or UGA. The coding regions tend to be stabilised by internal base pairs, this impedes degradation.<ref>{{cite journal |author=Shabalina SA, Ogurtsov AY, Spiridonov NA |title=A periodic pattern of mRNA secondary structure created by the genetic code |journal=Nucleic Acids Res. |volume=34 |issue=8 |pages=2428–37 |year=2006 |pmid=16682450 |pmc=1458515 |doi=10.1093/nar/gkl287}}</ref><ref>{{cite journal |author=Katz L, Burge CB |title=Widespread selection for local RNA secondary structure in coding regions of bacterial genes |journal=Genome Res. |volume=13 |issue=9 |pages=2042–51 |year=2003 |month=September |pmid=12952875 |pmc=403678 |doi=10.1101/gr.1257503}}</ref> In addition to being protein-coding, portions of coding regions may serve as regulatory sequences in the [[pre-mRNA]] as [[exonic splicing enhancer]]s or [[exonic splicing silencer]]s. |
|||
=== Untranslated regions === |
|||
{{main|5' UTR|3' UTR}} |
|||
Untranslated regions (UTRs) are sections of the mRNA before the start codon and after the stop codon that are not translated, termed the [[five prime untranslated region]] (5' UTR) and [[three prime untranslated region]] (3' UTR), respectively. These regions are transcribed with the coding region and thus are [[exon]]ic as they are present in the mature mRNA. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, and [[translational efficiency]]. The ability of a UTR to perform these functions depends on the sequence of the UTR and can differ between mRNAs. |
|||
The stability of mRNAs may be controlled by the 5' UTR and/or 3' UTR due to varying affinity for RNA degrading enzymes called [[ribonuclease]]s and for ancillary proteins that can promote or inhibit RNA degradation. |
|||
Translational efficiency, including sometimes the complete inhibition of translation, can be controlled by UTRs. Proteins that bind to either the 3' or 5' UTR may affect translation by influencing the ribosome's ability to bind to the mRNA. [[microRNA| MicroRNA]]s bound to the [[3' UTR]] also may affect translational efficiency or mRNA stability. |
|||
Cytoplasmic localization of mRNA is thought to be a function of the 3' UTR. Proteins that are needed in a particular region of the cell can actually be translated there; in such a case, the 3' UTR may contain sequences that allow the transcript to be localized to this region for translation. |
|||
Some of the elements contained in untranslated regions form a characteristic [[secondary structure]] when transcribed into RNA. These structural mRNA elements are involved in regulating the mRNA. Some, such as the [[SECIS element]], are targets for proteins to bind. One class of mRNA element, the [[riboswitch]]es, directly bind small molecules, changing their fold to modify levels of transcription or translation. In these cases, the mRNA regulates itself. |
|||
==== Splicing ==== |
|||
{{main|Splicing (genetics)}} |
|||
Splicing is the process by which pre-mRNA is modified to remove certain stretches of non-coding sequences called [[intron]]s; the stretches that remain include protein-coding sequences and are called [[exon]]s. Sometimes pre-mRNA messages may be spliced in several different ways, allowing a single gene to encode multiple proteins. This process is called [[alternative splicing]]. Splicing is usually performed by an RNA-protein complex called the [[spliceosome]], but some RNA molecules are also capable of catalyzing their own splicing (''see [[ribozyme]]s''). |
|||
====Editing==== |
|||
In some instances, an mRNA will be [[RNA editing|edited]], changing the nucleotide composition of that mRNA. In humans is the [[apolipoprotein B]] mRNA, which is edited in some tissues, but not others. The editing creates an early stop codon, which upon translation, produces a shorter protein. |
|||
==== Polyadenylation ==== |
|||
{{main|Polyadenylation}} |
|||
Polyadenylation is the covalent linkage of a polyadenylyl moiety to a messenger RNA molecule. Most messenger RNA (mRNA) molecules are polyadenylated at the 3' end. The [[messenger RNA#Poly(A) tail|poly(A) tail]] and the protein bound to it aid in protecting mRNA from degradation by exonucleases. Polyadenylation is also important for transcription termination, export of the mRNA from the nucleus, and translation. mRNA can also be polyadenylated in prokaryotic organisms, where poly(A) tails act to facilitate, rather than impede, exonucleolytic degradation. The 3' poly(A) tail is a long sequence of [[adenine]] nucleotides (often several hundred) at the [[3' end]] of the pre-mRNA. This tail promotes export from the nucleus and translation, and protects the mRNA from degradation. |
|||
=== Monocistronic versus polycistronic mRNA === |
|||
An mRNA molecule is said to be monocistronic when it contains the genetic information to [[Translation (genetics)|translate]] only a single [[protein]]. This is the case for most of the [[Eukaryote|eukaryotic]] mRNAs<ref name="Kozak_1983"> |
|||
{{ cite journal |
|||
| author = Kozak, M. |
|||
| year = 1983 |
|||
| month = March |
|||
| title = Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles |
|||
| journal = Microbiological Reviews |
|||
| volume = 47 |
|||
| issue = 1 |
|||
| pages = 1–45 |
|||
| doi = |
|||
| pmid = 6343825 |
|||
| url = http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=281560&blobtype=pdf |
|||
| format = PDF |
|||
| accessdate = 2006-08-12 |
|||
}} |
|||
</ref>. |
|||
==Elongation== |
|||
[[Image:simple transcription elongation1.svg|thumb|400px|Simple diagram of transcription elongation]] |
|||
One strand of DNA, the ''template strand'' (or non-coding strand), is used as a template for RNA synthesis. As transcription proceeds, RNA polymerase II traverses the template strand and uses base pairing complementarity with the DNA template to create an RNA copy. Although polymerase traverses the template strand from 3' → 5', the coding (non-template) strand is usually used as the reference point, so transcription is said to go from 5' → 3'. This produces an RNA molecule from 5' → 3', an exact copy of the coding strand (except that [[thymine]]s are replaced with [[uracil]]s, and the nucleotides are composed of a ribose (5-carbon) sugar where DNA has deoxyribose (one less oxygen atom) in its sugar-phosphate backbone). |
|||
mRNA transcription can involve multiple RNA polymerases on a single DNA template and multiple rounds of transcription (amplification of particular mRNA), so many mRNA molecules can be produced from a single copy of a gene. This step also involves a proofreading mechanism that can replace incorrectly incorporated bases. |
|||
The polymerase can experience pauses. These pauses may be intrinsic to RNA polymerase II or due to chromatin structure. Often the polymerase pauses to allow appropriate RNA editing factors to bind. |
|||
==Termination== |
|||
[[Image:simple transcription termination1.svg|thumb|400px|Simple diagram of transcription termination]] |
|||
Transcription termination in eukaryotes is less well understood, but involves cleavage of the new transcript, followed by template-independent addition of ''A''s at its new 3' end, in a process called [[polyadenylation]]. |
|||
Polyadenylation occurs during and immediately after transcription of DNA into RNA. After transcription has been terminated, the mRNA chain is cleaved through the action of an endonuclease complex associated with RNA polymerase II. After the mRNA has been cleaved, around 250 adenosine residues are added to the free 3' end at the cleavage site. This reaction is catalyzed by polyadenylate polymerase. Just as in alternative splicing, there can be more than one polyadenylation variant of a mRNA. |
|||
Once completely processed, the mRNA is termed ''[[mature mRNA]]''. |
|||
== Structure == |
|||
[[Image:MRNA structure.png|thumb|700px|center|The structure of a mature eukaryotic mRNA. A fully processed mRNA includes a [[5' cap]], [[5' UTR]], [[coding region]], [[3' UTR]], and poly(A) tail.]] |
|||
=Transport= |
|||
Because eukaryotic transcription and translation is compartmentally separated, eukaryotic mRNAs are exported from the [[cell nucleus|nucleus]] to the [[cytoplasm]]. Mature mRNAs are recognized by their processed modifications and then exported through the [[nuclear pore]]. |
|||
=Translation= |
|||
{{main|Translation (genetics)}} |
|||
Eukaryotic mRNA that has been processed and transported to the cytoplasm (i.e. mature mRNA) can then be translated by the ribosome. Translation may occur at [[ribosomes]] free-floating in the cytoplasm, or directed to the [[endoplasmic reticulum]] by the [[signal recognition particle]]. |
|||
=[[Post-translational modification]]= |
|||
This may include the formation of [[disulfide bridge]]s or attachment of any of a number of biochemical [[functional group]]s, such as [[acetate]], [[phosphate]], various [[lipid]]s and [[carbohydrate]]s. [[Enzyme]]s may also remove one or more amino acids from the leading (amino) end of the polypeptide chain, leaving a protein consisting of two polypeptide chains connected by disulfide bonds. |
|||
=[[Protein folding]]= |
|||
During and after synthesis, polypeptide chains often fold to assume, so called, native [[secondary structure|secondary]] and [[tertiary structure]]s. |
|||
=Degradation= |
|||
After a certain amount of time, the message is degraded by [[RNase]]s. The limited lifetime of mRNA enables a cell to alter protein synthesis rapidly in response to its changing needs. |
|||
Different mRNAs within the same cell have distinct lifetimes (stabilities). In mammalian cells, mRNA lifetimes range from several minutes to days. The greater the stability of an mRNA, the more protein may be produced from that mRNA. The presence of [[AU-rich element]]s in some mammalian mRNAs tends to destabilize those transcripts through the action of cellular proteins that bind these motifs. Rapid mRNA degradation via [[AU-rich element]]s is a critical mechanism for preventing the overproduction of potent cytokines such as tumor necrosis factor (TNF) and granulocyte-macrophage colony stimulating factor (GM-CSF).<ref name="Shaw1986">{{cite journal |author=Shaw G, Kamen R |title=A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation |journal=Cell |volume=46 |issue=5 |pages=659–67 |year=1986 |month=August |pmid=3488815 |doi=10.1016/0092-8674(86)90341-7}}</ref> Base pairing with a small interfering RNA ([[siRNA]]) or microRNA ([[miRNA]]) can also accelerate mRNA degradation. |
|||
=References= |
|||
{{Reflist|2}} |
|||
==Further reading== |
|||
* Doolittle, R.F. (1989) Redundancies in protein sequences. In ''Predictions of Protein Structure and the Principles of Protein Conformation'' (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623 |
|||
* David L. Nelson and Michael M. Cox, ''Lehninger Principles of Biochemistry'', 3rd edition, 2000, Worth Publishers, ISBN 1-57259-153-6 |
|||
* [[Uwe Meierhenrich]], ''Amino acids and the asymmetry of life'', Springer-Verlag, 2008, ISBN 978-3-540-76885-2 |
|||
==See also== |
|||
*[[Genetic code]] |
|||
*[[Cistron]] |
|||
*[[Operon]] |
|||
*[[lac operon|''lac'' operon]] |
|||
==External links== |
|||
* [http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=translation&rid=cooper.section.1167#1178 Translation of mRNA] Section of ''The Cell: A Molecular Approach'' by Geoffrey M. Cooper |
|||
* [http://www.scienceaid.co.uk/biology/genetics2/proteinsynthesis.html Science aid:Protein synthesis] For high school |
|||
* [http://www.accessexcellence.org/AB/GG/protein_synthesis.html Protein Synthesis] |
|||
* [http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/T/Transcription.html Transcription] |
|||
*: [http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/T/Translation.html Translation] |
|||
* [http://learningobjects.wesleyan.edu/proteinsynthesis/ Protein Synthesis Animation] Wesleyan University Learning Objects animation of protein synthesis. |
|||
* [http://cmol.nbi.dk/models/dynamtrans/dynamtrans.html Interactive Java simulation of transcription initiation.] From [http://cmol.nbi.dk/ Center for Models of Life] at the Niels Bohr Institute. |
|||
* From RNA to Protein Synthesis [http://www.asterpix.com/console/?avi=7698391 hypervideo]. |
|||
*[http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=stryer.TOC&depth=2 NCBI Bookshelf Free Textbook Access] |
|||
* [http://www.peptideguide.com/amino-acids/index.html Amino acids overview at Peptide Guide] |
|||
* [http://www.chem.qmul.ac.uk/iupac/AminoAcid/ Nomenclature and Symbolism for Amino Acids and Peptides] International Union of Pure and Applied Chemistry and The International Union of Biochemistry and Molecular Biology. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) |
|||
* [http://www.unc.edu/~bzafer/aminoacids/ListOfStandardAminoAcids.pdf The PDF List of Standard Amino Acids] |
|||
* [http://micro.magnet.fsu.edu/aminoacids/index.html Molecular Expressions: The Amino Acid Collection] - Has detailed information and microscopy photographs of each amino acid. |
|||
* [http://researchnews.osu.edu/archive/aminoacd.htm 22nd amino acid] - Press release from Ohio State describing the discovery of pyrrolysine as a 22nd amino acid. |
|||
* [http://www.russell.embl.de/aas/ Amino acid properties] - Properties of the amino acids (a tool aimed mostly at molecular geneticists trying to understand the meaning of mutations) |
|||
* [http://www.organic-chemistry.org/synthesis/C1C/nitrogen/alpha-amino-acids2.shtm Synthesis of Amino Acids and Derivatives] |
|||
* [http://www.newscientist.com/article.ns?id=mg19025545.200&feedId=online-news_rss20 Right-handed amino acids were left behind] |
|||
* [http://www2.iq.usp.br/docente/gutz/Curtipot_.html Amino acid solutions pH, titration curves and distribution diagrams - freeware] |
|||
* [http://isoelectric.ovh.org Protein isoelectric point according amino acids charge] |
|||
* [http://www.mathiasbader.de/studium/biology/index.php?lng=en Learn the 20 proteinogenic amino acids online] |
|||
{{AminoAcids}} |
|||
{{Protein primary structure}} |
|||
{{protein biosynthesis}} |
|||
{{Protein topics}} |
|||
{{Protein metabolism}} |
|||
{{Amino acid metabolism enzymes}} |
|||
{{metabolism-stub}} |
|||
[[Category:Gene expression]] |
|||
[[Category:Proteins]] |
|||
[[Category:Chemical synthesis]] |
|||
[[Category:Metabolism]] |
|||
[[Category:Protein biosynthesis]] |
|||
Revision as of 22:13, 10 October 2008
Biosynthesis of a Human Protein
| Human Lineage | |
|---|---|
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Subdivision: | |
| Subphylum: | |
| Infraphylum: | |
| Class: | |
| Infraclass: | |
| Superorder: | |
| Order: | |
| Suborder: | |
| Infraorder: | |
| Parvorder: | É. Geoffroy, 1812
|
| Family: | |
| Subfamily: | |
| Tribe: | |
| Subtribe: | |
| Genus: | Linnaeus, 1758
|
| Species: | sapiens
|
| Subspecies: | sapiens
Homo sapiens sapiens
|
Consider starting with a specific gene. Assemble the transcription mechanism at the appropriate genome location. Transcribe the gene using nucleotide accountability.
Protein biosynthesis
Alpha-amino acids are the building blocks of proteins. Amino acids combine in a condensation reaction (through dehydration synthesis) that releases water and the new "amino acid residue" held together by a peptide bond. Proteins are defined by their unique sequence of amino acid residues; this sequence is the primary structure of the protein.
Amino acid synthesis
Biochemical processes (metabolic pathways) produce amino acids from other compounds. The substrates for these processes are various compounds in a Human's diet or are produced resident flora in the gut. Humans are only able to synthesise 12 of the 20 standard amino acids.
Amino acids are synthesized from Glutamate, which is formed by amination of α-ketoglutarate:
Afterwards, Alanine and Aspartate are formed by transamination of Glutamate. All of the remaining amino acids are then constructed from Glutamate or Aspartate, by transamination of these two amino acids with one α-keto acid.
Synthesis from Intermediates of the Citric Acid Cycle and Other Major Pathways
Human beings can synthesize 11 (nonessential) of the basic set of 20 amino acids. The essential amino acids, which must be supplied in the diet. The pathways for the synthesis of nonessential amino acids are quite simple. Glutamate dehydrogenase catalyzes the reductive amination of α-ketoglutarate to glutamate. A transamination reaction takes place in the synthesis of most amino acids. At this step, the chirality of the amino acid is established. Alanine and aspartate are synthesized by the transamination of pyruvate and oxaloacetate, respectively. Glutamine is synthesized from NH4+ and glutamate, and asparagine is synthesized similarly. Proline and arginine are derived from glutamate. Serine, formed from 3-phosphoglycerate, is the precursor of glycine and cysteine. Tyrosine is synthesized by the hydroxylation of phenylalanine, an essential amino acid. The pathways for the biosynthesis of essential amino acids are much more complex than those for the nonessential ones.
Tetrahydrofolate, a carrier of activated one-carbon units, plays an important role in the metabolism of amino acids and nucleotides. This coenzyme carries one-carbon units at three oxidation states, which are interconvertible: most reduced—methyl; intermediate—methylene; and most oxidized—formyl, formimino, and methenyl. The major donor of activated methyl groups is S-adenosylmethionine, which is synthesized by the transfer of an adenosyl group from ATP to the sulfur atom of methionine. S-Adenosylhomocysteine is formed when the activated methyl group is transferred to an acceptor. It is hydrolyzed to adenosine and homocysteine, the latter of which is then methylated to methionine to complete the activated methyl cycle.
Nutritional importance
Of the 20 standard proteinogenic amino acids, 8 are called essential amino acids because the human body cannot synthesize them from other compounds at the level needed for normal growth, so they must be obtained from food.[1] However, the situation is a little more complicated since cysteine, tyrosine, histidine and arginine are semiessential amino acids in children, because the metabolic pathways that synthesize these amino acids are not fully developed.[2] The amounts required also depend on the age and health of the individual, so it is hard to make general statements about the dietary requirement for some amino acids.
| Essential | Nonessential |
|---|---|
| Isoleucine | Alanine |
| Leucine | Asparagine |
| Lysine | Aspartate |
| Methionine | Cysteine* |
| Phenylalanine | Glutamate |
| Threonine | Glutamine* |
| Tryptophan | Glycine* |
| Valine | Proline* |
| Serine* | |
| Tyrosine* | |
| Arginine* | |
| Histidine* |
(*) Essential only in certain cases.[3][4]
Regulation of Biosynthesis by Feedback Inhibition
Most of the pathways of amino acid biosynthesis are regulated by feedback inhibition, in which the committed step is allosterically inhibited by the final product. Branched pathways require extensive interaction among the branches that includes both negative and positive regulation. The regulation of glutamine synthetase from E. coli is a striking demonstration of cumulative feedback inhibition and of control by a cascade of reversible covalent modifications.
Transcription
A DNA sequence is enzymatically copied by RNA polymerase II to produce a complementary nucleotide RNA strand of messenger RNA (mRNA). mRNA is modified through RNA splicing, 5' end capping, and the addition of a polyA tail.[5]
Transcriber
In human biochemistry a transcriber might be thought of as a tool assembled in the nucleus for the transcription of genetic information. It synthesizes (a nucleic acid, usually RNA) using an existing nucleic acid (DNA) as a template from which to copy.
Transcription factors
Transcription factors bind to specific sequences of DNA and are part of the system that controls the transcription of genetic information from DNA to RNA.[6][7] Transcription factors occur in a complex, increase (as an activator), or prevent (as a repressor) the presence of RNA polymerase II.[8][9][10]
Each transcription factor contains one or more DNA binding domains (DBDs) which attach to specific sequences of DNA adjacent to the gene that it regulates.[11][12]
There are approximately 2600 proteins in the human genome that contain DNA-binding domains and most of these are presumed to function as transcription factors.[13]
The combinatorial use of a subset of the approximately 2000 human transcription factors easily accounts for the unique regulation of each gene in the human genome during development.[14]
General transcription factors (GTFs) are necessary for transcription to occur. GTFs are part of the large transcription preinitiation complex that interacts with RNA polymerase II directly.[15] They are ubiquitous and interact with the core promoter region surrounding the transcription start site(s) of allclass II genes.[16]
TFIIA TFIIB TFIID (see also TATA binding protein) TFIIE TFIIF TFIIH
Active transcription units (transcription factors) are clustered in the nucleus. There are ~10,000 factors in the nucleoplasm of a HeLa cell, among which are ~8,000 polymerase II factors and ~2,000 polymerase III factors. Each polymerase II factor contains ~8 polymerases. As most active transcription units are associated with only one polymerase, each factor will be associated with ~8 different transcription units. These units might be associated through promoters and/or enhancers, with loops forming a ‘cloud’ around the factor.
Additional proteins: coactivators chromatin remodelers histone acetylases deacetylases kinases methylases
play crucial roles in gene regulation.[14]
RNA polymerase II
Each of the RNA polymerases transcribes different classes of proteins. Specifically, RNA polymerase I transcribes rRNA and RNA polymerase III transcribes various additional RNAs.
RNA polymerase II transcribes Class II genes to form proteins. It catalyzes the transcription of DNA to synthesize precursors of mRNA and most snRNA and microRNA.[17]
It is a 550 kDa complex of 12 subunits.
Template:PBB Polymerase (RNA) II (DNA directed) polypeptide A, 220kDa, also known as POLR2A, is a human gene.
Template:PBB Polymerase (RNA) II (DNA directed) polypeptide B, 140kDa, also known as POLR2B, is a human gene.
Template:PBB The TATA binding protein (TBP) is a transcription factor that binds specifically to a DNA sequence called the TATA box. This DNA sequence is found about 25-30 base pairs upstream of the transcription start site in some eukaryotic gene promoters.[18] TBP, along with a variety of TBP-associated factors, make up the TFIID, a general transcription factor that in turn makes up part of the RNA polymerase II preinitiation complex.[10] As one of the few proteins in the preinitation complex that binds DNA in a sequence-specific manner, it helps position RNA polymerase II over the transcription start site of the gene. However, it is estimated that only 10-20% of human promoters have TATA boxes. Therefore, TBP is probably not the only protein involved in positioning RNA polymerase II.
TBP is a subunit of the eukaryotic transcription factor TFIID. TFIID is the first protein to bind to DNA during the formation of the pre-initiation transcription complex of RNA polymerase II (RNA Pol II). Binding of TFIID to the TATA box in the promoter region of the gene initiates the recruitment of other factors required for RNA Pol II to begin transcription. Some of the other recruited transcription factors include TFIIA, TFIIB and TFIIF. Each of these transcription factors are formed from the interaction of many protein subunits, indicating that transcription is a heavily regulated process.
When TBP binds to a TATA box within the DNA, it distorts the DNA by inserting amino acid side chains between base pairs, partially unwinding the helix, and doubly kinking it. The distortion is accomplished through a great amount of surface contact between the protein and DNA. TBP binds with the negatively charged phosphates in the DNA backbone through positively charged lysine and arginine amino acid residues. The sharp bend in the DNA is produced through projection of four bulky phenylalanine residues into the minor groove. As the DNA bends, its contact with TBP increases, thus enhancing the DNA-protein interaction.
The strain imposed on the DNA through this interaction initiates melting, or separation, of the strands. Because this region of DNA is rich in adenine and thymine residues, which base pair through only two hydrogen bonds, the DNA strands are more easily separated. Separation of the two strands exposes the bases and allows RNA polymerase II to begin transcription of the gene.
Initiation Regulation
Initiation is regulated by several mechanisms:
- Protein interference.
- Chromatin structure inhibition.
- Phosphorylation.
Regulation by Protein interference
Protein interference is the process where some signaling protein interacts, either with the promoter or some stage of the partially constructed complex, to prevent further construction of the polymerase complex. This is generally a very rapid response and is used for fine level, individual gene control and for 'cascade' processes for a group of genes useful under a specific conditions (for example DNA repair genes or heat shock genes).
Regulation by Chromatin structure inhibition
Chromatin structure inhibition is the process where the promoter is hidden by chromatin structure. Chromatin structure is controlled by post-translational modification of the histones involved and leads to gross levels of high or low transcription levels. See: chromatin, histone and nucleosome.
These methods of control can be combined in a modular method, allowing very high specificity in transcription initiation control.
Regulation by Phosphorylation
The largest subunit of Pol II (Rpb1) has a domain at its C-terminus that is called the CTD (C-terminal domain). This is the target of kinases and phosphatases. The phosphorylation of the CTD is an important regulation mechanism, as this allows attraction and rejection of factors that have a function in the transcription process. The CTD can be considered as a platform for transcription factors.
The CTD consists of repetitions of an amino acid motif, YSPTSPS, of which Serines and Threonines can be phosphorylated. The mammalian protein contains 52. There are many different combinations of phosphorylations possible on these repeats and these can change rapidly during transcription. The regulation of these phosphorylations and the consequences for the association of transcription factors plays a major role in the regulation of transcription.
During the transcription cycle, the CTD of the large subunit of RNAP II is reversibly phosphorylated. RNAP II containing unphosphorylated CTD is recruited to the promoter, whereas the hyperphosphorylated CTD form is involved in active transcription. Phosphorylation occurs at two sites within the heptapeptide repeat, at Serine 5 and Serine 2. Serine 5 phosphorylation is confined to promoter regions and is necessary for the initiation of transcription, whereas Serine 2 phosphorylation is important for mRNA elongation and 3'-end processing.
Mediator (coactivator)
Messenger RNA
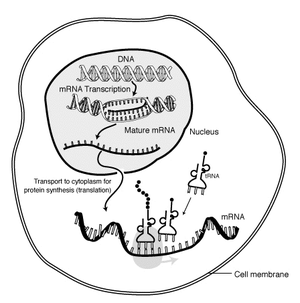
In the ribosome, the nucleic acid polymer is translated into a polymer of amino acids: a protein.
Pre-Initiation
RNA polymerase II binds to the DNA and, along with other cofactors, unwinds the DNA to create an initiation bubble so that the polymerase has access to the single-stranded DNA template. RNA Polymerase II does require a promoter like sequence.
Proximal (core) Promoters: TATA promoters are found around -30 bp to the start site of transcription. Not all genes have TATA box promoters and there exists TATA-less promoters as well. The TATA promoter consensus sequence is TATA(A/T)A(A/T).
The following are the steps involved in TATA Promoter Complex formation:
1. General transcription factors bind
2. TFIID, TFIIA, TFIIB, TFIIF (w/RNA Polymerase II), TFIIH/E
From the beginning of complex formation, the pre-initiation complex is closed.
Initiation

A collection of transcription factors mediate the binding of RNA polymerase II and the initiation of transcription. Only after certain transcription factors are attached to the promoter does the polymerase bind to it. The completed assembly of transcription factors and RNA polymerase II (transcription initiation complex) bind to the promoter.
Once the complex is opened by TFIIH initiation starts and the first phosphodiester bond is formed.
Promoter Clearance
During the formation of the first nucleotide bond the RNA polymerase begins to clear the promoter. There is a tendency to release the RNA transcript and produce truncated transcripts (abortive initiation). Once the transcript reaches approximately 23 nucleotides it no longer slips and Elongation can occur. This is an ATP dependent process. The short-lived, unprocessed or partially processed, product is termed pre-mRNA.
Promoter clearance also coincides with Phosphorylation of serine 5 on the carboxy terminal domain which is phosphorylated by TFIIH.
Eukaryotic pre-mRNA processing
Pre-mRNA requires extensive processing.
5' cap addition
A 5' cap (also termed an RNA cap, an RNA 7-methylguanosine cap or an RNA m7G cap) is a modified guanine nucleotide that is added to the "front" or 5' end of a the pre-mRNA using a 5',5-Triphosphate linkage shortly after the start of transcription. The 5' cap consists of a terminal 7-methylguanosine residue which is linked through a 5'-5'-triphosphate bond to the first transcribed nucleotide. Its presence is critical for recognition and proper attachment of mRNA by the ribosome and protection from 5' exonucleases RNases.
Cap addition is coupled to transcription, and occurs co-transcriptionally, such that each influences the other. Shortly after the start of transcription, the 5' end of the mRNA being synthesized is bound by a cap-synthesizing complex associated with RNA polymerase. This enzymatic complex catalyzes the chemical reactions that are required for mRNA capping. Synthesis proceeds as a multi-step biochemical reaction.
Coding regions
Coding regions are composed of codons, which are decoded and translated into one protein by the ribosome. Coding regions begin with the start codon and end with the a stop codons. Generally, the start codon is an AUG triplet and the stop codon is UAA, UAG, or UGA. The coding regions tend to be stabilised by internal base pairs, this impedes degradation.[19][20] In addition to being protein-coding, portions of coding regions may serve as regulatory sequences in the pre-mRNA as exonic splicing enhancers or exonic splicing silencers.
Untranslated regions
Untranslated regions (UTRs) are sections of the mRNA before the start codon and after the stop codon that are not translated, termed the five prime untranslated region (5' UTR) and three prime untranslated region (3' UTR), respectively. These regions are transcribed with the coding region and thus are exonic as they are present in the mature mRNA. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, and translational efficiency. The ability of a UTR to perform these functions depends on the sequence of the UTR and can differ between mRNAs.
The stability of mRNAs may be controlled by the 5' UTR and/or 3' UTR due to varying affinity for RNA degrading enzymes called ribonucleases and for ancillary proteins that can promote or inhibit RNA degradation.
Translational efficiency, including sometimes the complete inhibition of translation, can be controlled by UTRs. Proteins that bind to either the 3' or 5' UTR may affect translation by influencing the ribosome's ability to bind to the mRNA. MicroRNAs bound to the 3' UTR also may affect translational efficiency or mRNA stability.
Cytoplasmic localization of mRNA is thought to be a function of the 3' UTR. Proteins that are needed in a particular region of the cell can actually be translated there; in such a case, the 3' UTR may contain sequences that allow the transcript to be localized to this region for translation.
Some of the elements contained in untranslated regions form a characteristic secondary structure when transcribed into RNA. These structural mRNA elements are involved in regulating the mRNA. Some, such as the SECIS element, are targets for proteins to bind. One class of mRNA element, the riboswitches, directly bind small molecules, changing their fold to modify levels of transcription or translation. In these cases, the mRNA regulates itself.
Splicing
Splicing is the process by which pre-mRNA is modified to remove certain stretches of non-coding sequences called introns; the stretches that remain include protein-coding sequences and are called exons. Sometimes pre-mRNA messages may be spliced in several different ways, allowing a single gene to encode multiple proteins. This process is called alternative splicing. Splicing is usually performed by an RNA-protein complex called the spliceosome, but some RNA molecules are also capable of catalyzing their own splicing (see ribozymes).
Editing
In some instances, an mRNA will be edited, changing the nucleotide composition of that mRNA. In humans is the apolipoprotein B mRNA, which is edited in some tissues, but not others. The editing creates an early stop codon, which upon translation, produces a shorter protein.
Polyadenylation
Polyadenylation is the covalent linkage of a polyadenylyl moiety to a messenger RNA molecule. Most messenger RNA (mRNA) molecules are polyadenylated at the 3' end. The poly(A) tail and the protein bound to it aid in protecting mRNA from degradation by exonucleases. Polyadenylation is also important for transcription termination, export of the mRNA from the nucleus, and translation. mRNA can also be polyadenylated in prokaryotic organisms, where poly(A) tails act to facilitate, rather than impede, exonucleolytic degradation. The 3' poly(A) tail is a long sequence of adenine nucleotides (often several hundred) at the 3' end of the pre-mRNA. This tail promotes export from the nucleus and translation, and protects the mRNA from degradation.
Monocistronic versus polycistronic mRNA
An mRNA molecule is said to be monocistronic when it contains the genetic information to translate only a single protein. This is the case for most of the eukaryotic mRNAs[21].
Elongation

One strand of DNA, the template strand (or non-coding strand), is used as a template for RNA synthesis. As transcription proceeds, RNA polymerase II traverses the template strand and uses base pairing complementarity with the DNA template to create an RNA copy. Although polymerase traverses the template strand from 3' → 5', the coding (non-template) strand is usually used as the reference point, so transcription is said to go from 5' → 3'. This produces an RNA molecule from 5' → 3', an exact copy of the coding strand (except that thymines are replaced with uracils, and the nucleotides are composed of a ribose (5-carbon) sugar where DNA has deoxyribose (one less oxygen atom) in its sugar-phosphate backbone).
mRNA transcription can involve multiple RNA polymerases on a single DNA template and multiple rounds of transcription (amplification of particular mRNA), so many mRNA molecules can be produced from a single copy of a gene. This step also involves a proofreading mechanism that can replace incorrectly incorporated bases.
The polymerase can experience pauses. These pauses may be intrinsic to RNA polymerase II or due to chromatin structure. Often the polymerase pauses to allow appropriate RNA editing factors to bind.
Termination

Transcription termination in eukaryotes is less well understood, but involves cleavage of the new transcript, followed by template-independent addition of As at its new 3' end, in a process called polyadenylation.
Polyadenylation occurs during and immediately after transcription of DNA into RNA. After transcription has been terminated, the mRNA chain is cleaved through the action of an endonuclease complex associated with RNA polymerase II. After the mRNA has been cleaved, around 250 adenosine residues are added to the free 3' end at the cleavage site. This reaction is catalyzed by polyadenylate polymerase. Just as in alternative splicing, there can be more than one polyadenylation variant of a mRNA.
Once completely processed, the mRNA is termed mature mRNA.
Structure

Transport
Because eukaryotic transcription and translation is compartmentally separated, eukaryotic mRNAs are exported from the nucleus to the cytoplasm. Mature mRNAs are recognized by their processed modifications and then exported through the nuclear pore.
Translation
Eukaryotic mRNA that has been processed and transported to the cytoplasm (i.e. mature mRNA) can then be translated by the ribosome. Translation may occur at ribosomes free-floating in the cytoplasm, or directed to the endoplasmic reticulum by the signal recognition particle.
Post-translational modification
This may include the formation of disulfide bridges or attachment of any of a number of biochemical functional groups, such as acetate, phosphate, various lipids and carbohydrates. Enzymes may also remove one or more amino acids from the leading (amino) end of the polypeptide chain, leaving a protein consisting of two polypeptide chains connected by disulfide bonds.
Protein folding
During and after synthesis, polypeptide chains often fold to assume, so called, native secondary and tertiary structures.
Degradation
After a certain amount of time, the message is degraded by RNases. The limited lifetime of mRNA enables a cell to alter protein synthesis rapidly in response to its changing needs.
Different mRNAs within the same cell have distinct lifetimes (stabilities). In mammalian cells, mRNA lifetimes range from several minutes to days. The greater the stability of an mRNA, the more protein may be produced from that mRNA. The presence of AU-rich elements in some mammalian mRNAs tends to destabilize those transcripts through the action of cellular proteins that bind these motifs. Rapid mRNA degradation via AU-rich elements is a critical mechanism for preventing the overproduction of potent cytokines such as tumor necrosis factor (TNF) and granulocyte-macrophage colony stimulating factor (GM-CSF).[22] Base pairing with a small interfering RNA (siRNA) or microRNA (miRNA) can also accelerate mRNA degradation.
References
- ^ Young VR (1994). "Adult amino acid requirements: The case for a major revision in current recommendations" (PDF). J. Nutr. 124 (8 Suppl): 1517S–1523S. PMID 8064412.
- ^ Imura K, Okada A (1998). "Amino acid metabolism in pediatric patients". Nutrition. 14 (1): 143–8. doi:10.1016/S0899-9007(97)00230-X. PMID 9437700.
- ^ Fürst P, Stehle P (2004). "What are the essential elements needed for the determination of amino acid requirements in humans?". J. Nutr. 134 (6 Suppl): 1558S–1565S. PMID 15173430.
- ^ Reeds PJ (2000). "Dispensable and indispensable amino acids for humans". J. Nutr. 130 (7): 1835S–40S. PMID 10867060.
- ^ Robert J. Brooker Genetics: analysis and principles. 2nd edition. (New York: McGraw-Hill 2005) Chapter 12 "Gene transcription and RNA modification" pp. 318-325.
- ^ Latchman DS (1997). "Transcription factors: an overview". Int. J. Biochem. Cell Biol. 29 (12): 1305–12. doi:10.1016/S1357-2725(97)00085-X. PMID 9570129.
- ^ Karin M (1990). "Too many transcription factors: positive and negative interactions". New Biol. 2 (2): 126–31. PMID 2128034.
- ^ Roeder RG (1996). "The role of general initiation factors in transcription by RNA polymerase II". Trends Biochem. Sci. 21 (9): 327–35. doi:10.1016/0968-0004(96)10050-5. PMID 8870495.
- ^ Nikolov DB, Burley SK (1997). "RNA polymerase II transcription initiation: a structural view". Proc. Natl. Acad. Sci. U.S.A. 94 (1): 15–22. doi:10.1073/pnas.94.1.15. PMID 8990153.
- ^ a b Lee TI, Young RA (2000). "Transcription of eukaryotic protein-coding genes". Annu. Rev. Genet. 34: 77–137. doi:10.1146/annurev.genet.34.1.77. PMID 11092823.
- ^ Mitchell PJ, Tjian R (1989). "Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins". Science. 245 (4916): 371–8. doi:10.1126/science.2667136. PMID 2667136.
- ^ Ptashne M, Gann A (1997). "Transcriptional activation by recruitment". Nature. 386 (6625): 569–77. doi:10.1038/386569a0. PMID 9121580.
- ^ Babu MM, Luscombe NM, Aravind L, Gerstein M, Teichmann SA (2004). "Structure and evolution of transcriptional regulatory networks". Curr. Opin. Struct. Biol. 14 (3): 283–91. doi:10.1016/j.sbi.2004.05.004. PMID 15193307.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Brivanlou AH, Darnell JE (2002). "Signal transduction and the control of gene expression". Science. 295 (5556): 813–8. doi:10.1126/science.1066355. PMID 11823631.
- ^ Thomas MC, Chiang CM (2006). "The general transcription machinery and general cofactors". Critical reviews in biochemistry and molecular biology. 41 (3): 105–78. PMID 16858867.
- ^ Orphanides G, Lagrange T, Reinberg D (1996). "The general transcription factors of RNA polymerase II". Genes Dev. 10 (21): 2657–83. doi:10.1101/gad.10.21.2657. PMID 8946909.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kronberg, R.D. (1999). "Eukaryotic transcriptional control". Trends Cell Biol. 9 (12): M46–49. doi:10.1016/S0962-8924(99)01679-7. PMID 10611681.
- ^ Kornberg RD (2007). "The molecular basis of eukaryotic transcription". Proc. Natl. Acad. Sci. U.S.A. 104 (32): 12955–61. doi:10.1073/pnas.0704138104. PMID 17670940.
- ^ Shabalina SA, Ogurtsov AY, Spiridonov NA (2006). "A periodic pattern of mRNA secondary structure created by the genetic code". Nucleic Acids Res. 34 (8): 2428–37. doi:10.1093/nar/gkl287. PMC 1458515. PMID 16682450.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Katz L, Burge CB (2003). "Widespread selection for local RNA secondary structure in coding regions of bacterial genes". Genome Res. 13 (9): 2042–51. doi:10.1101/gr.1257503. PMC 403678. PMID 12952875.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^
Kozak, M. (1983). "Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles" (PDF). Microbiological Reviews. 47 (1): 1–45. PMID 6343825. Retrieved 2006-08-12.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Shaw G, Kamen R (1986). "A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation". Cell. 46 (5): 659–67. doi:10.1016/0092-8674(86)90341-7. PMID 3488815.
{{cite journal}}: Unknown parameter|month=ignored (help)
Further reading
- Doolittle, R.F. (1989) Redundancies in protein sequences. In Predictions of Protein Structure and the Principles of Protein Conformation (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623
- David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry, 3rd edition, 2000, Worth Publishers, ISBN 1-57259-153-6
- Uwe Meierhenrich, Amino acids and the asymmetry of life, Springer-Verlag, 2008, ISBN 978-3-540-76885-2
See also
External links
- Translation of mRNA Section of The Cell: A Molecular Approach by Geoffrey M. Cooper
- Science aid:Protein synthesis For high school
- Protein Synthesis
- Transcription
- Protein Synthesis Animation Wesleyan University Learning Objects animation of protein synthesis.
- Interactive Java simulation of transcription initiation. From Center for Models of Life at the Niels Bohr Institute.
- From RNA to Protein Synthesis hypervideo.
- NCBI Bookshelf Free Textbook Access
- Amino acids overview at Peptide Guide
- Nomenclature and Symbolism for Amino Acids and Peptides International Union of Pure and Applied Chemistry and The International Union of Biochemistry and Molecular Biology. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)
- The PDF List of Standard Amino Acids
- Molecular Expressions: The Amino Acid Collection - Has detailed information and microscopy photographs of each amino acid.
- 22nd amino acid - Press release from Ohio State describing the discovery of pyrrolysine as a 22nd amino acid.
- Amino acid properties - Properties of the amino acids (a tool aimed mostly at molecular geneticists trying to understand the meaning of mutations)
- Synthesis of Amino Acids and Derivatives
- Right-handed amino acids were left behind
- Amino acid solutions pH, titration curves and distribution diagrams - freeware
- Protein isoelectric point according amino acids charge
- Learn the 20 proteinogenic amino acids online


