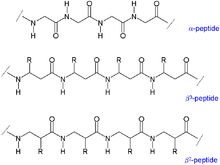
β peptides
β-peptides are synthetic peptides in which, in contrast to biological peptides, the peptide bond is on the β - carbon atom . β-peptides belong to the peptide mimetics .
properties
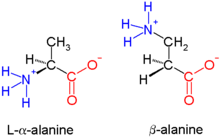
Biological peptides are α-peptides and, unlike β-peptides, are linked at the α- C atom - they consist of α- amino acids . The only known naturally occurring β-amino acid is β-alanine . In contrast to other amino acids, glycine cannot produce a β-amino acid due to the lack of a third carbon atom. β-peptides are more stable than α-peptides towards proteolysis and are being investigated for medical use.
In the course of a peptide synthesis of β-peptides, the chirality of the β-C atom must be preserved for biological activity . In contrast to the β-carbon atom of alanine, many β-carbon atoms of other amino acids are chiral. In the case of β-peptides, the side chains of the amino acids can be attached to the α- (β 3 -peptide) or the β-carbon atom (β 2 -peptide). Because of the three carbon atoms is a repeating unit of the peptide backbone in β-peptides to a carbon-carbon bond takes longer and altered secondary structures on, preferably in the gauche - conformation with respect to the C-C bond. Helical structures in β-peptides generally have a more stable conformation than in α-peptides. So far, different helical structures of β-peptides have been described, named after the number of atoms in a ring structure closed by a hydrogen bond in a helix: 8-helix, 10-helix, 12-helix, 14-helix and 10/12 helix. Likewise were folder structures described for β-peptides.
Various syntheses have been described, including those based on the Arndt-Eistert homologation . As the β-amino acid, β-alanine, β- leucine , β- lysine , β- arginine , β- glutamic acid , β- glutamine , β- phenylalanine and β- tyrosine were produced.
history
β-peptides were first described in 1996 by two working groups, around Dieter Seebach and Samuel Gellman .
literature
- RP Cheng, SH Gellman, WF DeGrado: beta-Peptides: from structure to function. In: Chemical Reviews . Volume 101, Number 10, October 2001, pp. 3219-3232, PMID 11710070 .
Individual evidence
- ↑ T. Beke, C. Somlai, A. Perczel: Toward a rational design of beta-peptide structures. In: Journal of computational chemistry. Volume 27, Number 1, January 2006, pp. 20-38, doi : 10.1002 / jcc.20299 , PMID 16247761 .
- ↑ EA Porter, B. Weisblum, SH Gellman: Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. In: Journal of the American Chemical Society . Volume 124, Number 25, June 2002, pp. 7324-7330, PMID 12071741 .
- ↑ Dieter Seebach, Jennifer L. Matthews: β-Peptides: a surprise at every turn. In: Chemical Communications. , S. 2015, doi : 10.1039 / a704933a .
- ^ K. Gademann, T. Hintermann, JV Schreiber: Beta-peptides: twisting and turning. In: Current medicinal chemistry. Volume 6, Number 10, October 1999, pp. 905-925, PMID 10519905 .
- ^ YD Wu, W. Han, DP Wang, Y. Gao, YL Zhao: Theoretical analysis of secondary structures of beta-peptides. In: Accounts of chemical research. Volume 41, Number 10, October 2008, pp. 1418-1427, doi : 10.1021 / ar800070b , PMID 18828608 .
- ↑ WF DeGrado, JP Schneider, Y. Hamuro: The twists and turns of beta-peptides. In: The journal of peptide research: official journal of the American Peptide Society. Volume 54, Number 3, September 1999, pp. 206-217, PMID 10517158 .
- ^ TA Martinek, F. Fülöp: Side-chain control of beta-peptide secondary structures. In: European Journal of Biochemistry . Volume 270, Number 18, September 2003, pp. 3657-3666, PMID 12950249 .
- ↑ B. Basler, O. Schuster, T. Bach: Conformationally constrained beta-amino acid derivatives by intramolecular [2 + 2] -photocycloaddition of a tetronic acid amide and subsequent lactone ring opening. In: The Journal of organic chemistry. Volume 70, Number 24, November 2005, pp. 9798-9808, doi : 10.1021 / jo0515226 , PMID 16292808 .
- ^ JK Murray, B. Farooqi, JD Sadowsky, M. Scalf, WA Freund, LM Smith, J. Chen, SH Gellman: Efficient synthesis of a beta-peptide combinatorial library with microwave irradiation. In: Journal of the American Chemical Society . Volume 127, Number 38, September 2005, pp. 13271-13280, doi : 10.1021 / ja052733v , PMID 16173757 .
- ↑ MJ Koyack, RP Cheng: Design and synthesis of beta-peptides with biological activity. In: Methods in molecular biology. Volume 340, 2006, pp. 95-109, doi : 10.1385 / 1-59745-116-9: 95 , PMID 16957334 .
- ↑ Eusebio Juaristi, Vadim A. Soloshonok: Enantioselective Synthesis of β ‐ Amino Acids. John Wiley & Sons, 2005, ISBN 9780471467380 .
- ↑ Dieter Seebach, Mark Overhand, Florian NM Kühnle, Bruno Martinoni, Lukas Oberer, Ulrich Hommel, Hans Widmer: β-Peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a β-hexapeptide in solution and its stability towards pe. In: Helvetica Chimica Acta. 79, 1996, p. 913, doi : 10.1002 / hlca.19960790402 .
- Jump up ↑ Daniel H. Appella, Laurie A. Christianson, Isabella L. Karle, Douglas R. Powell, Samuel H. Gellman: β-Peptide Foldamers: Robust Helix Formation in a New Family of β-Amino Acid Oligomers. In: Journal of the American Chemical Society. 118, 1996, p. 13071, doi : 10.1021 / ja963290l .