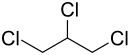
1,2,3-trichloropropane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,2,3-trichloropropane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 Cl 3 | |||||||||||||||
| Brief description |
colorless, not very volatile liquid with a chloroform- like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 147.43 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.39 g cm −3 |
|||||||||||||||
| Melting point |
−14.7 ° C |
|||||||||||||||
| boiling point |
156 ° C |
|||||||||||||||
| Vapor pressure |
2.8 h Pa (20 ° C) |
|||||||||||||||
| solubility |
heavy in water (1.75 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.484 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic, toxic for reproduction ( CMR ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−230.6 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,2,3-Trichloropropane is a chemical compound from the group of saturated chlorinated hydrocarbons . It is in the form of a liquid that is difficult to ignite.
Extraction and presentation
1,2,3-Trichloropropane is obtained by chlorinating propene , but it also occurs as a by-product in the synthesis of other chlorinated compounds (e.g. epichlorohydrin ). In the year 2000 the USA produced about 9000-14,000 tons, worldwide less than 50,000 tons per year.
use
1,2,3-Trichloropropane is used as a solvent for oils, fats, waxes and especially polymers , as well as a trifunctional crosslinker (e.g. in polysulphide elastomers ) and for the synthesis of heterocycles .
safety instructions
1,2,3-Trichloropropane is classified as carcinogenic (Category 1B), toxic to reproduction (Category 1A) and germ cell mutagenic (Category 2), as well as, according to Annex II, No. 6 of the German Hazardous Substances Ordinance (GefStoffV), as a particularly dangerous carcinogenic substance are only manufactured or used in closed systems.
See also
Isomers :
- 1,1,2-Trichloropropane , CAS No .: 598-77-6
- 1,1,3-trichloropropane , CAS-No .: 20395-25-9
- 1,2,2-Trichloropropane , CAS No .: 3175-23-3
Web links
- Entry on 1,2,3-trichloropropane in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ a b c d e f g h i Entry on 1,2,3-trichloropropane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Data sheet 1,2,3-trichloropropane from Sigma-Aldrich , accessed on March 5, 2011 ( PDF ).
- ↑ Entry on 1,2,3-trichloropropane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 17, 2014.
- ↑ Data sheet 1,2,3-trichloropropane (PDF) from Merck , accessed on January 19, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ^ Concise International Chemical Assessment Document (CICAD) for 1,2,3-TRICHLOROPROPANE , accessed November 18, 2014.
- ↑ Hazardous Substances Ordinance (GefStoffV) - as of April 2017 .


