2,4,6-tris (biphenyl-4-yl) -1,3,5-triazine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,4,6-tris (biphenyl-4-yl) -1,3,5-triazine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 39 H 27 N 3 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 537.66 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
284 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,4,6-Tris (biphenyl-4-yl) -1,3,5-triazine is a chemical compound that acts as a UV filter and is used in sunscreens . It is a broadband UV filter that offers protection against UVAII and UVB radiation with wavelengths from 290 to 340 nanometers .
With the 2,4,6-tris (biphenyl-3-yl) -1,3,5-triazine and the 2,4,6-tris (biphenyl-2-yl) -1,3,5-triazine still exist two isomeric trisbiphenyltriazines .
Extraction and presentation
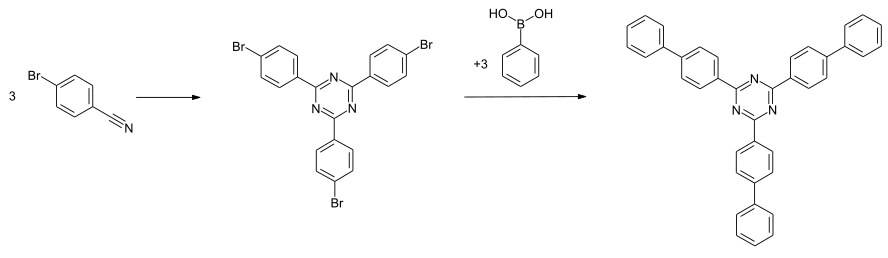
The synthesis can be carried out by trimerization of 4-bromobenzonitrile and subsequent coupling of the intermediate 2,4,6-tris (bromophen-4-yl) -1,3,5-triazine with benzene boronic acid in a Suzuki reaction in the presence of a palladium catalyst .
Further manufacturing options are the trimerization of biphenyl-4-carbonitrile in the presence of aluminum chloride and the reaction of cyanuric chloride with biphenyl, likewise in the presence of aluminum chloride.
Trade names
- Tinosorb A2B, ETH50
Web links
- SCCS ( Scientific Committee on Consumer Safety ), Opinion on 1,3,5-triazine, 2,4,6-tris (1,1'-biphenyl) -4-yl- , September 20, 2011
Individual evidence
- ↑ SCCS (Scientific Committee on Consumer Safety), Opinion on 1,3,5-triazine, 2,4,6-tris (1,1'-biphenyl) -4-yl- , September 20, 2011.
- ↑ a b Tsutomu Ishi-i, Kentaro Yaguma, Thies Thiemann, Masataka Yashima, Kazunori Ueno, Shuntaro Mataka: High Electron Drift Mobility in an Amorphous Film of 2,4,6, -Tris [4- (1-naphthyl) phenyl] -1,3,5-triazines . In: Chemistry Letters . tape 33 , no. 10 , 2004, p. 1244-1245 , doi : 10.1246 / cl.2004.1244 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ innovadex.eu: Tinosorb® A2B ( Memento from February 23, 2014 in the Internet Archive ), accessed on May 2, 2019.
- ↑ Patent application US6225467 : Electroluminescent (EL) devices. Applied on January 21, 2000 , published on May 1, 2001 , Applicants: Xerox , Inventors: Mohammad Esteghamatian, Nan-Xing Hu, Zoran D. Popovic, Ah-Mee Hor, Beng S. Ong.
- ↑ patent application WO2004085412A2 : Symetrical triazines Derivatives. Registered on March 24, 2003 , published on July 29, 2003 , applicant: Ciba Specialty Chemicals , inventors: Thomas Ehlis, Stefan Müller, Pascal Hayoz.
- ↑ Entry on 2,4,6-tris (biphenyl-4-yl) -1,3,5-triazine in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 20, 2020.