2,5-dimethoxy-4-methylphenylethylamine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
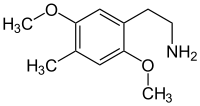
|
|||||||||||||
| General | |||||||||||||
| Surname | 2,5-dimethoxy-4-methylphenylethylamine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 11 H 17 NO 2 | ||||||||||||
| Brief description |
white crystals (hydrochloride) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 195.26 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
213-214 ° C (hydrochloride) |
||||||||||||
| solubility |
good in ethanol , acetonitrile and isopropanol |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
2,5-dimethoxy-4-methylphenylethylamin (also abbreviated 2C-D ) is a psychedelic effective hallucinogen , which due to its structure to the substance groups of the phenol (including alkyl aryl ether ) of phenethylamine , and the 2Cs counts. The substance was developed in 1970 by a group at the Texas Research Institute of Mental Sciences . The American pharmacologist Alexander Shulgin then described the synthesis and effects on humans in his book PiHKAL . Among other things, 2C-D acts as a potent agonist of the serotonin receptors 5-HT 2A / 2C . 2C-D was used under Hanscarl Leuner in psycholytic psychotherapy as LE-25.
Legal status
In Germany, 2C-D has been subject to the Narcotics Act since the 28th ordinance amending the regulations on narcotics.
literature
- Pablo R. Moya, Kelly A. Berg, Manuel A. Gutiérrez-Hernandez, Patricio Sáez-Briones, Miguel Reyes-Parada, Bruce K. Cassels, William P. Clarke: "Functional Selectivity of Hallucinogenic Phenethylamine and Phenylisopropylamine Derivatives at Human 5- Hydroxytryptamine (5-HT) 2A and 5-HT 2C Receptors ", in: Journal of Pharmacology and Experimental Therapeutics , 2007 , 321 (3) , 1054-1061; doi: 10.1124 / jpet.106.117507 .
- Robert T. Standridge, Henry G. Howell, Jonas A. Gylys, Richard A. Partyka, Alexander T. Shulgin: "Phenylalkylamines with potential psychotherapeutic utility. 1. 2-Amino-1- (2,5-dimethoxy-4-methylphenyl ) butane ", in: Journal of Medicinal Chemistry , 1976 , 19 (12) , 1400-1404; doi: 10.1021 / jm00234a010 .
- Beng-Thong Ho, L. Wayne Tansey, Robert L. Balster, Rong An, William M. McIsaac, Robert T. Harris: "Amphetamine analogs. II. Methylated phenethylamines", in: Journal of Medicinal Chemistry , 1970 , 13 (1 ) , 134-135; doi: 10.1021 / jm00295a034 .
Web links
Individual evidence
- ↑ a b c PiHKAL # 23 2C-D
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on 2,5-dimethoxy-4-methyl-phenethylamine in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ Ho BT, Tansey LW, Balster RL, An R, McIsaac WM, Harris RT: Amphetamine analogs. II. Methylated phenethylamines . In: Journal of Medicinal Chemistry . 13, No. 1, January 1970, pp. 134-5. doi : 10.1021 / jm00295a034 . PMID 5412084 .
- ↑ CA Villalobos, P. Bull, P. Sáez, BK Cassels, JP Huidobro-Toro: 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT 2A receptor antagonists in Xenopus laevis oocytes , in: Br. J. Pharmacol. , 2004 , 141 (7) , 1167-1174; PMC 1574890 (free full text).
- ↑ Pablo R. Moya, Kelly A. Berg, Manuel A. Gutiérrez-Hernandez, Patricio Sáez-Briones, Miguel Reyes-Parada, Bruce K. Cassels, William P. Clarke: "Functional Selectivity of Hallucinogenic Phenethylamine and Phenylisopropylamine Derivatives in Human 5 -Hydroxytryptamine (5-HT) 2A and 5-HT 2C Receptors ", in: Journal of Pharmacology and Experimental Therapeutics , 2007 , 321 (3) , 1054-1061; doi: 10.1124 / jpet.106.117507 .
- ^ Peyton Jacob III and Alexander T. Shulgin: Structure-Activity Relationships of the Classic Hallucinogens and Their Analogs ( Memento of July 23, 2015 in the Internet Archive ). In: NIDA Research Monograph 146: Hallucinogens: An Update. Pp. 74, 1994, United States Department of Health and Human Services.
- ↑ Erowid Character Vaults: Hanscarl Leuner
- ^ DG Addiction Medicine (PDF).