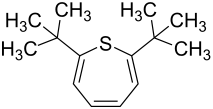
2,7-di- tert- butylthiepine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | 2,7-di- tert- butylthiepine | |||||||||
| Molecular formula | C 14 H 22 S | |||||||||
| Brief description |
colorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 222.39 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.08 g cm −3 |
|||||||||
| Melting point |
36-36.5 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
2,7-Di- tert- butylthiepine is a heterocyclic chemical compound . It consists of a thieping skeleton , on the 2- and 7-positions of which tert- butyl radicals are substituted .
Manufacturing
2,7-Di- tert- butylthiepine can be formed by ring expansion from a derivative of 2,6-di- tert- butylthiopyran. The compound used has a leaving group , which enables ring expansion. Acetic anhydride and sodium acetate are used for this reaction .

properties
It is a colorless solid that melts at 36 ° C. The compound is in a trough conformation in which four carbon atoms form a plane. It has a system of eight π electrons and is one of the anti-aromatic compounds. Most thiepines are unstable compounds that decompose to benzene derivatives by splitting off sulfur . 2,7-Di- tert- butylthiepin, however, is significantly more stable than thiepin itself. This is due to the steric hindrance caused by the bulging tert-butyl radicals. In boiling toluene , it has a half-life of 365 hours.
Reactions
2,7-di- tert -butylthiepin can be prepared by the reaction with triphenylphosphine to the synthesis of 1,2-di- tert -butylbenzene be used.
Furthermore, cycloadditions can be carried out, which is due to the antiaromatic character of the thiepin ring.
Individual evidence
- ↑ a b c d e K. Yamamoto, S. Yamazaki, Y. Kohashi, I. Murata, Y. Kai, in: Tetrahedron Lett. 1982 , 23 (31) , 3195-3198.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b R. Gleiter, G. Krennrich, D. Cremer, K. Yamamoto, I. Murata: Electronic Structure and Stability of Thiepins. Photoelectron spectroscopic investigation , in: J. Am. Chem. Soc. 1985 , 107 , 6874-6879.
- ↑ S. Yamazaki, A. Isokawa, K. Yamamoto, I. Murata, in: J. Chem. Soc., Perkin Trans. 1 1994 , 18 , 2631-2636.