Furan-2-carboxylic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
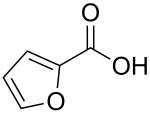
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Furan-2-carboxylic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 4 O 3 | |||||||||||||||
| Brief description |
beige powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 112.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
128-132 ° C |
|||||||||||||||
| boiling point |
230-232 ° C |
|||||||||||||||
| Vapor pressure |
1.8 hPa (96 ° C) |
|||||||||||||||
| pK s value |
3.16 (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Furan-2-carboxylic acid is a chemical compound from the group of furan derivatives and carboxylic acids .
Extraction and presentation
Furan-2-carboxylic acid can be obtained by oxidation of furfural (e.g. Cannizzaro reaction ).
It occurs as an intermediate in the Brenzung of mucus acid to furan and formed when frying food. The former was already noticed by Carl Wilhelm Scheele and Trommsdorf, who, however, did not see the product as an independent connection. It was not until 1818 that Labillardière recognized the pyrogenic acid in this.
properties
Furan-2-carboxylic acid is a colorless to beige powder that is sparingly soluble in water. The furan ring is stabilized by the carboxy group so that the compound can be nitrated, sulfonated, esterified (on the carboxy group) and converted into its acid chloride . When heated, the compound decarboxylates to form furan.
use
Furan-2-carboxylic acid is a metabolite of furfural (2-furylmethanal) and is analyzed as its biomarker in urine. Furan-2-carboxylic acid has bactericidal properties and, like benzoic acid, is used as a preservative . It also serves as a gloss agent in paint production . The esters of furan-2-carboxylic acid have a pleasant fruity smell and are used as a fragrance.
See also
Individual evidence
- ↑ a b c d e f g h data sheet 2-Furoic acid, 98% from Sigma-Aldrich , accessed on May 26, 2013 ( PDF ).
- ↑ a b Data sheet furan-2-carboxylic acid (PDF) from Merck , accessed on May 26, 2013.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-44.
- ^ A b c Karl-Heinz Lautenschläger: Pocket book of chemistry . Harri Deutsch Verlag, 2005, ISBN 978-3-8171-1760-4 , pp. 478 ( limited preview in Google Book search).
- ^ A b Raj K. Bansal: Heterocyclic Chemistry . New Age International, 1999, ISBN 81-224-1212-2 , pp. 188 ( limited preview in Google Book search).
- ^ Friedrich Klages : Textbook of organic chemistry. Vol. 1. Systematic organic chemistry. Half 2. Nitrogen and other non-metal compounds, organometallic compounds, cyclic compounds, etc. a. Walter de Gruyter, 1953, ISBN 3-11-157251-X , p. 892 ( limited preview in Google Book search).
- ↑ Peter Brandt: Reports on Food Safety 2007: Food Monitoring . Springer, 2008, ISBN 978-3-7643-8912-3 , pp. 64 ( limited preview in Google Book search).
- ^ Leopold Gmelin: Handbook of theoretical chemistry . In F. Varrentrapp, 1829, p. 176 ( limited preview in Google Book search).
- ^ Adalbert Wollrab: Organic chemistry . Springer DE, 2002, ISBN 978-3-540-43998-1 , pp. 849 ( limited preview in Google Book search).
- ↑ Furan-2-carboxylic acid and other carboxylic acids (phenylglyoxylic acid, mandelic acid, t, t-muconic acid, benzoic acid, hydroxybenzoic acids, hippuric acid, methylhippuric acids, 2,4-dichlorobenzoic acid, 3-methyl-4-nitro-benzoic acid, TTCA) [Biomonitoring Methods in German language, 2006] . In: The MAK Collection for Occupational Health and Safety . Wiley-VCH, Weinheim 2012, ISBN 978-3-527-60041-0 , doi : 10.1002 / 3527600418.bi8814d0017 .
