2-hydroxybutyric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
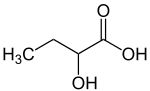
|
|||||||||||||||||||
| Structure without considering stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-hydroxybutyric acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 O 3 | ||||||||||||||||||
| Brief description |
white crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 104.10 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.125 g cm −3 (20 ° C, racemate) |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
260 ° C (racemate) |
||||||||||||||||||
| solubility |
soluble in water, ether and ethanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-Hydroxybutyric acid , systematically 2-hydroxybutanoic acid , is a chemical compound from the group of hydroxycarboxylic acids . Their salts are called 2-hydroxybutyrates.
Isomers
2-Hydroxybutyric acid contains a stereocenter , is therefore chiral and occurs in two enantiomeric forms , ( R ) -2-hydroxybutyric acid and ( S ) -2-hydroxybutyric acid. Racemic 2-hydroxybutyric acid [synonym: ( RS ) -2-hydroxybutyric acid] is a 1: 1 mixture of the ( R ) - and the ( S ) -enantiomer.
| Isomers of 2-hydroxybutyric acid | ||
| Surname | ( S ) -2-hydroxybutyric acid | ( R ) -2-hydroxybutyric acid |
| other names | (+) - 2-hydroxybutyric acid L -2-hydroxybutyric acid |
(-) - 2-Hydroxybutyric acid D -2-hydroxybutyric acid |
| Structural formula |

|

|
| CAS number | 3347-90-8 | 20016-85-7 |
| 600-15-7 (racemate) | ||
| EC number | 628-477-6 | 627-239-9 |
| 209-985-3 (racemate) | ||
| ECHA info card | 100.156.743 | 100.155.619 |
| 100.009.079 (racemate) | ||
| PubChem | 440864 | 449265 |
| 11266 (racemate) | ||
| Wikidata | Q27887417 | Q27122144 |
| Q3288610 (racemate) | ||
presentation
2-Hydroxybutyric acid can be made from 2-bromobutanoic acid and silver oxide .
Another synthesis starts from propanal , which is converted into the corresponding cyanohydrin with hydrogen cyanide . The hydrolysis of the nitrile group leads to 2-hydroxybenzoic acid with elimination of ammonia .
Heating ethyltartronic acid to 180 ° C also gives 2-hydroxybutanoic acid with decarboxylation .
See also
Individual evidence
- ↑ a b c d data sheet (R) -2-Hydroxybutyric acid from Sigma-Aldrich , accessed on January 7, 2013 ( PDF ).
- ^ A b D. R. Lide: CRC Handbook of Chemistry and Physics . CRC Press, 2012, ISBN 1-4398-8049-2 ( limited preview in Google Book Search).
- ↑ a b Entry on (S) -2-HYDROXYBUTYRIC ACID at ChemicalBook , accessed September 14, 2012.
- ^ A b c F. Beilstein: Handbook of organic chemistry , 3rd edition, 1st volume, Verlag Leopold Voss, 1893. P. 561 ( full text ).
- ↑ C. Friedel, V. Machuca: About bromobutyric acid and a new acid derived from it . In: Justus Liebigs Annalen der Chemie , 1861, 120 (3), pp. 279-285 ( doi : 10.1002 / jlac.18611200305 ).

