2-bromobutyric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of 2-bromobutanoic acid without specifying the stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-bromobutyric acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 7 BrO 2 | ||||||||||||||||||
| Brief description |
colorless, foul-smelling liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 167.0 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.57 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−4 ° C |
||||||||||||||||||
| boiling point |
99-103 ° C (13 h Pa ) |
||||||||||||||||||
| Vapor pressure |
11 Pa (25 ° C) |
||||||||||||||||||
| solubility |
moderate in water (66 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-bromobutyric acid ( 2-bromobutyric acid ) is one of the organic compounds and is an α-bromine derivative of butyric acid and one of the bromobutyric acids .
Stereoisomerism
2-Bromobutyric acid contains a stereocenter and is therefore chiral . Racemic 2-bromobutyric acid [synonym: ( RS ) -2-bromobutyric acid] is a 1: 1 mixture of ( R ) -2-bromobutyric acid and ( S ) -2-bromobutyric acid.
| Isomers of 2-bromobutanoic acid | ||
| Surname | ( S ) -2-bromobutanoic acid | ( R ) -2-bromobutanoic acid |
| other names | (-) - 2-bromobutanoic acid | (+) - 2-bromobutanoic acid |
| Structural formula |

|

|
| CAS number | 32659-49-7 | 2681-94-9 |
| 80-58-0 (racemate) | ||
| EC number | - | - |
| 201-294-5 (racemate) | ||
| ECHA info card | - | - |
| 100.001.177 (racemate) | ||
| PubChem | 638123 | 6992738 |
| 6655 (racemate) | ||
| Wikidata | Q27293869 | Q27251681 |
| Q209315 (racemate) | ||
Extraction and presentation
Racemic 2-bromobutyric acid is easy to prepare from butyric acid , elemental bromine and red phosphorus . This formation reaction is called the Hell-Volhard-Zelinsky reaction . The volatile esters are irritating to tears.
The pure enantiomers - ( R ) -2-bromobutyric acid and ( S ) -2-bromobutyric acid - can be prepared from ( RS ) -2-bromobutyric acid by resolution . This succeeded z. B. chromatographically on a chiral stationary phase.
Reactions
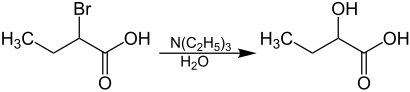
2-bromobutyric acid reacts with water in the presence of triethylamine to form 2-hydroxybutyric acid .
Reaction with aqueous NH 3 produces α-aminobutyric acid .
Individual evidence
- ↑ a b c d e f Data sheet 2-bromobutyric acid (PDF) from Merck , accessed on December 1, 2019.
- ↑ a b Data sheet 2-Bromobutyric acid from AlfaAesar, accessed on December 1, 2019 ( PDF )(JavaScript required) .
- ^ Organic-chemie.ch: Hell-Volhard-Zelinsky .
- ^ Daniel Armstrong, Advanced Separation Technologies, U.S. Patent No. 5154738, Priority Date: September 12, 1989.
- ^ F. Beilstein: Handbook of organic chemistry , 3rd edition, 1st volume. Verlag Leopold Voss, 1893. p. 483. Full text