2-iodobutane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Simplified structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-iodobutane | ||||||||||||||||||
| other names |
sec -butyl iodide |
||||||||||||||||||
| Molecular formula | C 4 H 9 I. | ||||||||||||||||||
| Brief description |
colorless liquid with an ethereal odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 184.02 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.59 g cm −3 |
||||||||||||||||||
| Melting point |
-104 ° C |
||||||||||||||||||
| boiling point |
118 ° C |
||||||||||||||||||
| Vapor pressure |
60 hPa (30 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.499 (20 ° C) 1.4991 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2-Iodobutane is a chemical compound from the group of aliphatic , saturated organic halogenated hydrocarbons .
Isomers
2-iodobutane occurs in two stereoisomeric forms, ( S ) -2-iodobutane and ( R ) -2-iodobutane.
| Isomers of 2-iodobutane | ||
| Surname | ( S ) -2-iodobutane | ( R ) -2-iodobutane |
| other names | (+) - 2-iodobutane | (-) - 2-iodobutane |
| Structural formula |

|
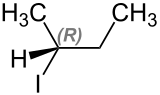
|
| CAS number | 29882-56-2 | 22156-92-9 |
| 513-48-4 (unspec.) | ||
| EC number | - | - |
| 208-163-1 (unspec.) | ||
| ECHA info card | - | - |
| 100.007.422 (unspec.) | ||
| PubChem | 12475337 | 12475336 |
| 10559 (unspec.) | ||
| Wikidata | Q27236239 | Q27277859 |
| Q27258588 (unspec.) | ||
Extraction and presentation
2-Iodobutane can be obtained by reacting 2-bromobutane with iodine ; another possibility is the Finkelstein reaction of sodium iodide with 2-bromobutane.
The reaction of ( R ) -2-bromobutane with sodium iodide in acetone produces ( S ) -2-iodobutane. If the racemate of 2-bromobutane is used as the starting product , then racemic 2-iodobutane is formed.
It is also possible to produce it by an addition reaction of hydrogen iodide with 1-butene .
properties
2-Iodobutane is a highly flammable, volatile, colorless liquid with an ethereal odor, which is practically insoluble in water.
use
2-Iodobutane is used as a solvent in organic synthesis and as an intermediate in pharmaceuticals.
safety instructions
The vapors of 2-iodobutane can form an explosive mixture with air ( flash point 21 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2-iodobutane in the GESTIS substance database of the IFA , accessed on January 27, 2019(JavaScript required) .
- ↑ a b c data sheet 2-iodobutane, 99%, stab. with copper at AlfaAesar, accessed on January 27, 2019 ( PDF )(JavaScript required) .
- ^ Kurt Peter C. Vollhardt, Neil Eric Schore: Organic Chemistry . John Wiley & Sons, 2011, ISBN 3-527-32754-1 , pp. 241 ( limited preview in Google Book search).
- ^ Alyn William Johnson: Invitation to Organic Chemistry . Jones & Bartlett Learning, 1999, ISBN 978-0-7637-0432-2 , pp. 93 ( limited preview in Google Book search).
- ^ Henry Rakoff, Norman C. Rose: Organic chemistry . Macmillan, 1966, pp. 98 ( limited preview in Google Book search).

