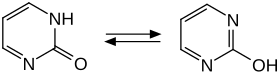
2-pyrimidinone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
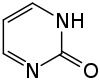
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-pyrimidinone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 4 N 2 O | ||||||||||||||||||
| Brief description |
light yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 96.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.37 g cm −3 |
||||||||||||||||||
| Melting point |
179-181 ° C |
||||||||||||||||||
| solubility |
very soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-pyrimidinone is a chemical compound from the group of pyrimidines . The connection corresponds to the basic structure of many nucleobases such as B. the cytosine .
Extraction and presentation
The compound can be obtained by the oxidation of 2-chloropyrimidine with hydrogen peroxide .
properties
2-pyrimidinone is a crystalline solid that occurs in a tetragonal crystal lattice with the space group P 4 1 2 1 2 (space group no. 92) . A tautomeric equilibrium can be formulated for the compound with a keto and an enol form. Quantum chemical calculations show that the keto structure is the more stable form.
Individual evidence
- ↑ Fargher, RG; Pyman, FL: XXVI.-Nitro-, arylazo-, and amino-glyoxalines in J. Chem. Soc. 115 (1919) 217-260, doi : 10.1039 / CT9191500217 .
- ↑ a b Furberg, S .; Solbakk, J .: Crystal Structure of Pyrimidine-2-one in Acta Chem. Scand. 24 (1970) 3230-3236, doi : 10.3891 / acta.chem.scand.24-3230 .
- ↑ Hunt, RR; McOmie, JFW; Sayer, ER: 109. Pyrimidines. Part X. Pyrimidine, 4: 6-dimethylpyrimidine, and their 1-oxides in J. Chem. Soc. 1959, 525-530, doi : 10.1039 / JR9590000525 .
- ↑ Albert, A .; Brown, DJ; Cheeseman, G .: 812. Pteridine studies. Part III. The solubility and the stability to hydrolysis of pteridines in J. Chem. Soc. 1952, 4219-4232, doi : 10.1039 / JR9520004219 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Cantrell Jr., WR; Bauta, WE; Engles, T .: Hydrogen peroxide promoted hydroxylation of haloarenes and heteroarenes in Tetrahedron Lett. 47 (2006) 4249-4251, doi : 10.1016 / j.tetlet.2006.04.020 .
- ↑ Les, A .; Adamowicz, L .: Theoretical ab initio study of the protomeric tautomerism of 2-hydroxypyrimidine, 4-hydroxypyrimidine, and their derivatives in J. Phys. Chem. 94 (1990) 7012-7032, doi : 10.1021 / j100381a020 .
- ↑ Cieplak, P .; Bash, P .; Singh, CU; Kollman, PA: A theoretical study of tautomerism in the gas phase and aqueous solution: a combined use of state-of-the-art ab initio quantum mechanics and free energy-perturbation methods in J. Am. Chem. Soc. 109 (1987) 6283-6289, doi : 10.1021 / ja00255a010 .