3-benzoxepine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | 3-benzoxepine | |||||||||
| Molecular formula | C 10 H 8 O | |||||||||
| Brief description |
lemon yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 144.17 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
|
|||||||||
| solubility |
soluble in non-polar solvents such as diethyl ether , benzene and carbon tetrachloride , and alcohols such as. B. methanol |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
3-Benzoxepine is a fused ring system consisting of an aromatic benzene ring and the non-aromatic, unsaturated, oxygen - containing seven - membered heterocycle oxepine , the synthesis of which was reported by Karl Dimroth and co-workers in 1961.
Occurrence
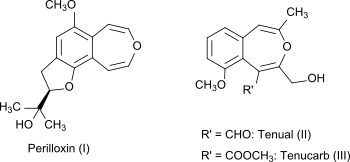
3-Benzoxepine does not occur naturally, but its bicyclic ring system is found in the natural substances Perilloxin (I) from Perilla ( Perilla frutescens ), Acuta and Tenual (II) or Tenucarb (III) from Asphodeline tenuior .
presentation
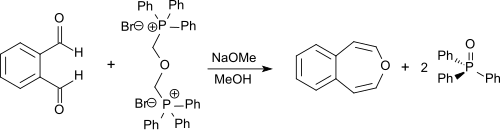
In the preparation of unsubstituted 3-benzoxepin by Karl Dimroth in a double is Wittig reaction phthalaldehyde α'-Dibromdimethylether [from α, bis (bromomethyl) ether (- (α'-α triphenylphosphonium,) dimethyl-dibromide with bis-, BBME), obtainable from hydrobromic acid and paraformaldehyde in 97% yield] and triphenylphosphine - obtained with sodium methoxide in dry methanol in 55% yield.
UV irradiation of naphthalene derivatives , such as. B. 1,4-epoxy-1,4-dihydronaphthalene
or photo-oxidation of 1,4-dihydronaphthalene and subsequent pyrolysis of the resulting hydroperoxide .
also provides 3-benzoxepine in small quantities (yields 4–6%).
properties
3-Benzoxepine is a solid that crystallizes in lemon-yellow flakes and resembles naphthalene in odor and easy sublimability . The substance dissolves in non-polar organic solvents and, like naphthalene, can be purified by sublimation. 3-Benzoxepine is relatively acid-stable; Only when boiling with concentrated alcoholic hydrochloric acid does an unsaturated aldehyde develop, which is probably indene- 3-aldehyde. Catalytic hydrogenation with palladium gives 1,2,4,5-tetrahydro-3-benzoxepine.
Chlorine and bromine are added to form undefined dihalides, from which 3-benzoxepine is recovered by zinc dust distillation.
Individual evidence
- ↑ a b c d K. Dimroth, G. Pohl: 3-Benzoxepin . In: Angew. Chem. Band 73 , no. 12 , 1961, pp. 436 , doi : 10.1002 / anie.19610731215 .
- ↑ a b c K. Dimroth, G. Pohl, H. Follmann: The synthesis of derivatives of 3-oxepine and furan by a twofold Wittig reaction . In: Chem. Ber. tape 99 , no. 2 , 1966, p. 634-641 , doi : 10.1002 / cber.19660990238 .
- ↑ a b A. Rosowsky: The Chemistry of Heterocyclic Compounds, Seven-membered Heterocyclic Compounds Containing Oxygen and Sulfur, 26th Volume II Oxepin ring system Containing Two ring. . Wiley-Interscience, New York 1972, ISBN 0-471-38210-8 , pp. 96 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ J. Liu, A. Steigel, E. Reininger, R. Bauer: Two New Prenylated 3-Benzoxepine Derivatives as Cyclooxygenase Inhibitors from Perilla frutescens var. Acuta . In: J. Nat. Prod. Band 63 , no. 3 , 2000, pp. 403-405 , doi : 10.1021 / np990362o .
- ↑ A. Ulubelen, E. Tuzlaci, N. Atilan: Oxepine derivatives and anthraquinones from Asphodeline tenuior and A. Taurica . In: Phytochem. tape 28 , no. 2 , 1989, pp. 649-650 , doi : 10.1016 / 0031-9422 (89) 80076-7 .
- ↑ JC Bill, DS Tarbell: o-Phthalaldehyde In: Organic Syntheses . 34, 1954, p. 82, doi : 10.15227 / orgsyn.034.0082 ; Coll. Vol. 4, 1963, p. 807 ( PDF ).
- ↑ Patent US20040242799A1 : Process to bromomethylate aromatic compounds. Filed August 29, 2001 , published December 2, 2004 , inventors: M. Grabarnick, Y. Sasson.
- ↑ a b G.R. Ziegler: Mechanisms of photochemical reactions in solution. LVII. Photo arrangement of 1,4-epoxy-1,4-dihydronaphthalene to benz [f] oxepin . In: J. Am. Chem. Soc. tape 91 , no. 2 , 1969, p. 446-449 , doi : 10.1021 / ja01030a040 .
- ↑ AM Jeffrey, DM Jerina: Autoxidation of 1,4-dihydronaphthalene. Formation of 3-benzoxepine via pyrolysis of 2-hydroperoxy-1,2-dihydronaphthalenes . In: J. Am. Chem. Soc. tape 94 , no. 11 , 1972, p. 4048-4049 , doi : 10.1021 / ja00766a084 .