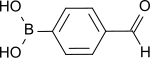
4-formylphenylboronic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-formylphenylboronic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 8 BO 3 | |||||||||||||||
| Brief description |
whitish powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 149.94 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point | ||||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
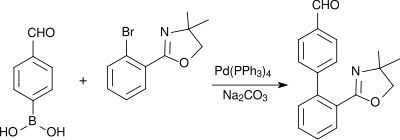
4-Formylphenylboronic acid ( 4-FPBA ) is a versatile synthetic building block and an important intermediate in the preparation of agrochemical and pharmaceutical active ingredients. The substance finds industrial applications as a stabilizer and inhibitor for enzymes and as a bactericide .
Occurrence and representation
The synthesis of 4-formylphenylboronic acid was reported in 1990 by Heinrich Nöth's group , using 4-bromobenzaldehyde as the starting material . The acetalization of the aldehyde group is carried out according to standard methods with triethyl orthoformate and ethanol to give 1-bromo-4- (diethoxymethyl) benzene. The formation of the Grignard compound with magnesium requires 1,2-dibromoethane and activation with ultrasound . Reaction with tri- n -butylborat leads to the protected Arylborsäureester, from the strength in acidic work-up in 78% yield the target product is obtained.
With the same reactants, but by activation with Red-Al and reaction with the boric acid ester at −60 ° C, 4-FPBA gives in 94% yield, even on a kilogram scale.
When using the aryllithium compound of 1-bromo-4- (diethoxymethyl) benzene with n-butyllithium instead of the Grignard compound at −78 ° C, 4-formylphenylboronic acid is obtained in 99% crude yield with triisopropylborate to introduce the boronic acid function.
Disadvantages of both routes are the high price of the starting materials used, such as 4-bromobenzaldehyde, boric acid esters with higher alcohols and butyllithium, and the difficult work-up after hydrolysis by n-butanol in the Nöth route.
More recently, an improved process using less expensive raw materials such as 4-chlorobenzaldehyde , metallic lithium and trimethyl borate has been patented.
4-Formylphenylboronic acid can also be prepared by the hydrolysis of potassium 4-formylphenyl trifluoroborate using acidic alumina or silica . As a rule, phenylboronic acids are used as starting compounds for the corresponding potassium aryl trifluoroborates.
properties
4-Formylphenylboronic acid crystallizes in colorless needles or is obtained as an odorless, whitish powder that dissolves little in cold, better in hot water. The compound is extremely stable and easily forms dimers and cyclic trimeric anhydrides , which make purification difficult and tend to protodeboronation , a side reaction that occurs frequently in the Suzuki coupling, with elimination of the boronic acid function.
Applications
4-Formylphenylboronic acid is used in Suzuki couplings to build up pharmacologically active biphenyl compounds, such as. B. with an improved synthesis of a precursor of the blood pressure lowering AT1 antagonist telmisartan .
Also palladium-catalyzed aryl-heteroaryl linkages by Suzuki use 4-FPBA as molecular building block such. B. in the synthesis of aryl benzimidazole derivatives that dock to peroxisome proliferator-activated receptors (PPARγ) and activate the expression of a large number of genes.
In a copper- mediated fluoroalkylation reaction, the boronic acid group of the 4-FPBA can be replaced by a perfluoroalkyl chain with perfluorinated alkyl iodides (R f -I) under mild conditions.
4-Formyphenylboronic acid is used on an industrial scale as an enzyme stabilizer for proteases and especially for lipases in liquid detergent preparations. The addition of 4-FPBA in amounts of <0.08 percent by weight in the formulation reduces the loss of hydrolytic activity of the enzymes used and increases the storage stability of enzyme-containing liquid detergents.
Individual evidence
- ↑ a b Data sheet 4-Formylphenylboronic acid from Sigma-Aldrich , accessed on December 10, 2016 ( PDF ).
- ↑ a b Data sheet 4-Formylbenzeneboronic acid from AlfaAesar, accessed on December 10, 2016 ( PDF )(JavaScript required) .
- ↑ a b c Entry on 4-formylphenylboronic acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b c H. Feulner, G. Linti, H. Nöth: Contributions to the chemistry of boron, 206. Representation and structural characterization of p-formylbenzene boronic acid . In: Chem. Ber. tape 123 , no. 9 , 1990, pp. 1841–1843 , doi : 10.1002 / cber.19901230915 .
- ↑ Entry on 4-formylphenylboronic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 20, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Patent US5972873 : 4-Substituted-phenyl-boronic acids as enzyme stabilizers. Applied November 21, 1997 , published October 26, 1999 , Applicant: Novo Nordisk A / S, Inventor: LK Nielsen, A. Deane-Wray.
- ^ Author collective, Organikum , 24th edition, p. 481, Wiley-VCH, Weinheim, 2001, ISBN 978-3-527-33968-6
- ↑ a b H. Jendralla, A. Wagner, M. Mollath, J. Wunner: Efficient, simple procedures for the large-scale preparation of buildings blocks for angiotensin (II) receptor antagonists . In: Liebigs Ann. Chem. Band 1995 , no. 7 , 1995, p. 1253-1257 , doi : 10.1002 / jlac.1995199507166 .
- ^ Y. Kobayashi, Y. Tokoro, K. Watatani: Preparation of functionalized zinc borates and their coupling reactions with allylic acetates . In: Tetrahedron Lett. tape 39 , no. 41 , 1998, pp. 7537-7540 , doi : 10.1016 / s-0040-4039 (98) 01639-6 .
- ↑ Patent US6833470B2 : Method for producing formylphenylboronic acids. Registered on November 30, 2001 , published on December 21, 2004 , applicant: Clariant GmbH, inventors: A. Meudt, S. Scherer, F. Vollmüller, M. Erbes.
- ↑ GW Kabalka, V. Coltuclu: Thermal and microwave hydrolysis of organotrifluoroborates mediated by alumina . In: Tetrahedron Lett. tape 50 , no. 46 , 2009, p. 6271-6272 , doi : 10.1016 / j.tetlet.2009.09.008 .
- ^ GA Molander, LN Cavalcanti, B. Canturk, P.-S. Pan, LE Kennedy: Efficient hydrolysis of organotrifluoroborates via silica gel and water . In: J. Org. Chem. Band 74 , no. 19 , 2009, p. 7364-7369 , doi : 10.1021 / jo901441u .
- ↑ E. Vedejs, RW Chapman, SC Fields, S. Lin, MR Schimpf: Conversion of arylboronic acids into aryltrifluoroborates: convenient precursors of arylboron difluoride Lewis acids . In: J. Org. Chem. Band 60 , no. 10 , 1995, p. 3020-3027 , doi : 10.1021 / jo00115a016 .
- ↑ GK Surya Prakash, F. Pertusati, GA Olah: HF-free, direct synthesis of tetrabutylammonium trifluoroborates . In: Synthesis . tape 2011 , no. 2 , 2011, p. 292-302 , doi : 10.1055 / s-0030-1258370 .
- ↑ AS Kumar, S. Ghosh, GN Mehta: Efficient and improved synthesis of Telmisartan . In: Beilstein J. Org. Chem. Volume 25 , 2010, p. 6 , doi : 10.3762 / bjoc.6.25 .
- ↑ G. Singh, A. Singh, V. Singh, RK Verma, R. Mall: Novel benzimidazole derivatives as partial PPARγ agonists: synthesis, characterization, and docking studies . In: WJPPS . tape 5 , no. 7 , 2016, p. 1080-1091 , doi : 10.20959 / wjpps20167-7143 .
- ↑ Q. Qi, Q. Shen, L. Lu: Copper-mediated aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides at room temperature . In: J. Am. Chem. Soc. tape 134 , no. 15 , 2012, p. 6548-6551 , doi : 10.1021 / ja301705z .
- ↑ Patent US20130252315A1 : Stabilized, liquid, enzyme-containing surfactant preparation. Registered on May 14, 2013 , published on September 26, 2013 , applicant: Henkel AG & Co. KGaA, inventor: T. O'Connell, S. Tondera, T. Weber.