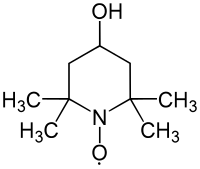
4-hydroxy-2,2,6,6-tetramethylpiperidinyloxyl
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxyl | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 9 H 18 NO 2 | |||||||||||||||||||||
| Brief description |
orange crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 172.24 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.127 g cm −3 |
|||||||||||||||||||||
| Melting point |
67-71 ° C |
|||||||||||||||||||||
| Vapor pressure |
<0.01 Pa (20 ° C) |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
4-Hydroxy-2,2,6,6-tetramethylpiperidinyloxyl ( 4-Hydroxy-TEMPO ) is a stabilized radical that can be used as an oxidizing agent . The reactivity of the compound is similar to that of 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO). The additional hydroxyl group allows a link to other compounds.
Extraction and presentation
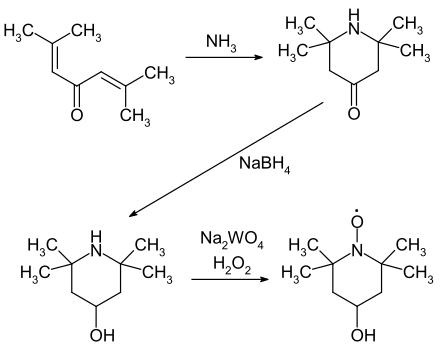
The synthesis is based on the Phoron , which can easily be obtained from acetone via a double aldol condensation . In the first step, cyclization to 2,2,6,6-tetramethyl-piperidin-4-one takes place in the presence of ammonia . This is then reduced to 2,2,6,6-tetramethyl-piperidin-4-ol using sodium borohydride . The target compound is obtained in the last step by oxidation with hydrogen peroxide in the presence of sodium tungstate .
properties
4-Hydroxy-2,2,6,6-tetramethylpiperidinyloxyl forms orange crystals that melt at 70 ° C. Decomposition is observed from 140 ° C.
use
The compound is used as an inhibitor in free radical polymerization reactions . It acts as a radical scavenger and regulator for a controlled polymerisation process. It can be used as a reaction stopper in continuous polymerization reactions. In organic synthesis, it acts as a catalytic oxidizing agent in the selective oxidation of primary alcohols to aldehydes in the presence of sodium hypochlorite or 1,4-benzoquinone . With activated hydrocarbons, such as cyclohexene , the substance reacts with hydrogen abstraction to form the corresponding radical intermediates, which recombine with further 4-hydroxy-TEMPO to form the N-alkoxyamine compound.
The OH functionality enables it to be incorporated into polymer chains, which results in polymer-based TEMPO materials. These can be used to oxidize alcohols to the corresponding aldehydes and ketones. A medical application as an antihypertensive agent was investigated in animal models.
Individual evidence
- ↑ Entry on HYDROXY TETRAMETHYLPIPERIDINE OXIDE in the CosIng database of the EU Commission, accessed on June 3, 2020.
- ↑ a b c d e f g Evonik Industries AG: GPS Safety Summary 4-HT , March 2014.
- ↑ a b c d e Entry on 4-Hydroxy-2,2,6,6-tetramethyl-1-piperidinyloxyl in e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons, Inc., accessed on February 10, 2016.
- ↑ a b entry to 4-hydroxy-2,2,6,6-tetramethylpiperidinoxyl in the GESTIS database of IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Ciriminna, R .; Pagliaro, M .: Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives in Org. Process Res. Dev. 14 (2010) 245-251, doi : 10.1021 / op900059x .
- ^ LF Fieser and M. Fieser, Textbook of Organic Chemistry, translated and edited by HR Hensel, p. 222, Verlag Chemie, Weinheim, 1954.
- ↑ Platkowski, K .; Reichert, K.-H .: Studies on stopping the radical polymerization of methyl methacrylate with the inhibitor 4-Hydroxytempo in Chem. Ing. Techn. 71 (1999) 493-496, doi : 10.1002 / cite.330710516 .
- ↑ Babiarz, JE; Cunkle, GT; DeBellis, A D .; Eveland, D .; Pastor, SD; Shum, SP: The Thermal Reaction of Sterically Hindered Nitroxyl Radicals with Allylic and Benzylic Substrates: Experimental and Computational Evidence for Divergent Mechanisms in J. Org. Chem. 67 (2002) 6831-6834, doi : 10.1021 / jo020426r .
- ↑ Vogler, T .; Studer, A .: Applications of TEMPO in Synthesis in Synthesis 2008, 1979-1993, doi : 10.1055 / s-2008-1078445 .
- ↑ Tanyeli, C .; Gumus, A .: Synthesis of polymer-supported TEMPO catalysts and their application in the oxidation of various alcohols in Tetrahedron Letters 44 (2003) 1639-1642, doi : 10.1016 / S0040-4039 (03) 00003-0 .
- ↑ Wilcox, CS; Pearlman, A .: Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides in Pharmacol. Rev. 60 (2008) 418-469, doi : 10.1124 / pr.108.000240 .