Alcuronium chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
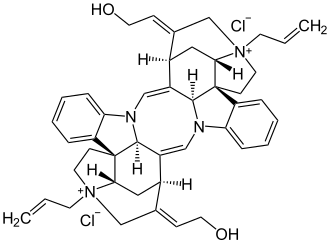
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Alcuronium chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 44 H 50 Cl 2 N 4 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 737.799 g mol −1 | |||||||||||||||
| Melting point |
> 350 ° C (from 220 ° C decomposition) |
|||||||||||||||
| boiling point |
decomposition |
|||||||||||||||
| solubility |
good in water and alcohols |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Alcuronium chloride ( Alcuronium ) is a semi-synthetic derivative of the alkaloid toxiferin , which is the main component of calebassen curare , and is therefore one of the Strychnos alkaloids . It is one of the non-depolarizing muscle relaxants.
properties
Alcuronium chloride is an odorless, colorless, crystalline powder. When heated, the substance changes color from around 220 ° C and does not melt up to 350 ° C. The optically active compound, which is soluble in water such as methanol and ethanol, shows a specific angle of rotation of −348 in methanol .
Clinical use
In clinical use, it is used for precurarization (dosage 0.03 mg / kg body weight ( body weight ) ) before the administration of depolarizing muscle relaxants (e.g. suxamethonium chloride ) in order to minimize muscle ache-like symptoms after anesthesia. When used alone, its dose is initially 0.15–0.25 mg / kg body weight and the maintenance dose is 0.02–0.05 mg / kg body weight. The effect occurs after 3–5 minutes and lasts between 60 and 80 minutes.
Side effects
The following side effects can be observed:
- Uncommon: cramping of the muscles that span the airways ( bronchospasm ); Cardiac arrhythmias and cardiac arrest as well as allergic skin reactions and gastrointestinal complaints .
- Very rare: drop in blood pressure associated with a continuously accelerated pulse ( tachycardia ); anaphylactic reactions as well as transient, mild arterial hypertension or increased pulse rate. Its use should be reserved for experienced anesthetists or emergency doctors .
Absorption and elimination
Since the chloride is completely ionized in the physiological pH range , it is hardly absorbed from the gastrointestinal tract or tissues and is therefore only used intravenously .
The substance is not metabolized in the organism. About 5% appears in the bile , while the majority is eliminated renally (through the kidneys).
proof
Contamination with diallyl caracurine (DAC) and the allyl Wieland Gumlich aldehyde (WCA) can be determined by means of capillary electrophoresis with a detection limit of less than 0.1%. The detection limit for Alcuronium using an HPLC method is 0.025 mg · l −1 plasma.
Individual evidence
- ^ Official version of the ATC index with DDD information for Germany in 2008 ( Memento from August 12, 2011 in the Internet Archive ) (PDF; 1.3 MB).
- ↑ a b c d H. HJ Hager, F. v. Bruchhausen: Hager's handbook of pharmaceutical practice. 3rd edition, Vol. 7 fabrics A – D, pp. 96–98, 1994, Birkhäuser-Verlag, ISBN 3-540-52688-9 .
- ↑ a b data sheet ALCURONIUM CHLORIDE CRS (PDF) at EDQM , accessed on July 17, 2008.
- ↑ a b Alcuronium chloride ( Memento from October 31, 2016 in the Internet Archive )
- ↑ a b Oyo Yakuri. Pharmacometrics. Vol. 3, p. 390, 1969.
- ↑ Gendai no Rinsho. Vol. 1, Pg. 349, 1967.
- ↑ K. Aktories, U. Förstermann, F. Hofmann, K. Starke: General and special pharmacology and toxicology. 2006, Elsevier-Verlag, ISBN 3-437-44490-5 .
- ↑ Summary of Product Alloferin ® ICN Pharmaceuticals Germany GmbH.
- ↑ Wedig, M. et al. : Evaluation of the impurity profile of alcuronium by means of capillary electrophoresis. in: J Pharm Biomed Anal . 2002; 28: 983-90, PMID 12039641 .
- ↑ Künzer, T. et al. : Simple and rapid high-performance liquid chromatography method for the determination of alcuronium in human plasma and urine. in: J. Chromatogr. B . 1994; 653: 63-68, PMID 8012561 .
Trade names
Alcuronium chloride is commercially available in Germany under the name Alloferin, but is rarely used today.
![{\ displaystyle \ left [a \ right] _ {D} ^ {22}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/523cd82cb5305e6ba6cb160997a30ca85a74e741)