Aluminum sodium sulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
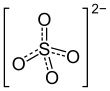
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum sodium sulfate | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.61 g cm −3 (dodecahydrate) |
||||||||||||||||||
| Melting point |
~ 60 ° C (dodecahydrate) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum sodium sulphate ( E 521 , sodium alum ) in the form of its dodecahydrate is an alum with the composition NaAl (SO 4 ) 2 · 12 H 2 O, which is used as a firming agent , acidifier and acid regulator .
Occurrence
Of course, aluminum sodium sulfate occurs as undecahydrate in the mineral mendocite NaAl (SO 4 ) 2 · 11 H 2 O.
properties
Aluminum sodium sulfate is a colorless solid and a mixed salt of sulfuric acid , which contains sodium and aluminum as metals .
safety instructions
The salt is irritating to the skin, eyes and mucous membranes and can cause eczema . It is suspected of causing allergic reactions and therefore frequent consumption is not recommended. In addition, it is suspected of causing Alzheimer's disease due to aluminum exposure of the body .
See also
Web links
- Additives Online: E 521 - Aluminum Sodium Sulphate
Individual evidence
- ↑ Entry on E 521: Aluminum sodium sulphate in the European database for food additives, accessed on June 27, 2020.
- ↑ Entry on SODIUM ALUM in the CosIng database of the EU Commission, accessed on February 12, 2020.
- ↑ a b c d e David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 83 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ List of additives and E numbers. In: bll.de. Federation for Food Law and Food Science , accessed on March 4, 2017 .
- ^ DC Ayres, D. Hellier: Dictionary of Environmentally Important Chemicals , 1998, CRC Press, ISBN 0-7514-0256-7 .
- ↑ Salzburg Chamber of Labor ( Memento from November 28, 2007 in the Internet Archive ) (PDF; 385 kB).