Aluminum tristearate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
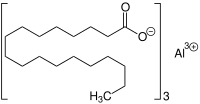
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum tristearate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 54 H 105 AlO 6 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 877.35 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.01 g cm −3 |
||||||||||||||||||
| Melting point |
105-115 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum tristearate is a chemical compound from the group of aluminum compounds and fatty acid salts ( metal soaps ). It is the aluminum salt of stearic acid .
Extraction and presentation
Aluminum tristearate can be obtained by reacting stearic acid with aluminum hydroxide or with aluminum triisopropoxide .
properties
Aluminum tristearate is a white flammable solid that is practically insoluble in water. The ignition temperature is 375 ° C.
use
Aluminum stearate is used as a thickening agent in many industries . It is used in lacquers, paints, printing inks and cleaning agents in suspensions to increase viscosity . It is also used as a matting agent additive in paints and varnishes . In rubber it is used in processing, and in the plastics industry it is included in topcoats for coated fabrics. It is also used in powder form for lubrication and sealing, as well as in the manufacture of anti-rust equipment and defoamers. It is also used for the hydrophobicity of absorbent materials such as paper, textiles and concrete . In medicine, it is also used as a gel former for oleogels and to increase viscosity.
Related links
- Aluminum distearate , C 36 H 71 AlO 5 (CAS No. 300-92-5)
Individual evidence
- ↑ a b c d e f g h Entry on aluminum tristearate in the GESTIS substance database of the IFA , accessed on December 22, 2019(JavaScript required) .
- ↑ a b MP Biomedicals: Aluminum stearate ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ^ A b Arnold Willmes: Pocket book chemical substances: Elements- Inorganika- Organika , ISBN 978-3-8171-1787-1 ; P. 80.
- ↑ F. von Bruchhausen, Hermann Hager: Hagers Handbook of Pharmaceutical Practice , p. 149.
