Aspartic acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Aspartic acid | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 4 H 6 O 2 S 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 150.22 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
76.5-77.5 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Aspartic acid is a sulphurous carboxylic acid found in common asparagus ( Asparagus officinalis ). This heterocyclic compound is a derivative of isobutyric acid and, in addition to the carboxy group, contains a disulfide group in the ring (1,2-dithiolane). The sulfur-containing breakdown products in the urine after consuming asparagus vegetables can develop a distinctive odor .
Occurrence
In vegetables asparagus asparagusic acid occurs naturally in addition to its reduced form, Dihydroasparagusinsäure (Dithiolisobuttersäure).
Aspartic acid has a growth-inhibiting effect comparable to the phytohormone abscisic acid and also has a nematicidal effect .
A noticeable odor of urine can often be noticed after consuming asparagus; it is attributed to volatile, sulphurous metabolites of aspartic acid . But not all people form the same breakdown products to the same extent and the ability to smell them in urine is also different.
metabolism
The breakdown of aspartic acid in the human body depends on the availability of enzymes . In many people, due to their genetic disposition in the asparagusin metabolism, the methyl esters 2-propenthioic acid S- methyl ester (thioacrylic acid S -methyl ester) and 3- (methylthio) thiopropionic acid S -methyl ester are formed by enzymatic cleavage . These metabolites play a special role in the subsequent development of the asparagus odor in urine.
Further degradation results in various volatile sulfur-containing compounds that occur in the urine; In particular, methanethiol (methyl mercaptan), dimethyl sulfide , dimethyl disulfide , 2,4-dithiapentane (bis (methylthio) methane), dimethyl sulfoxide and dimethyl sulfone are suspected to be important odor components.
Laboratory synthesis
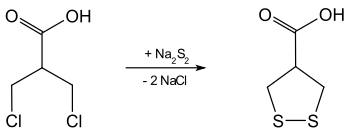
Aspartic acid can be prepared from β, β'-dichloroisobutyric acid and sodium disulfide ( accessible in situ from sodium sulfide and sulfur ).
Thermal degradation
When the asparagus is cooked , the aspartic acid is thermally broken down into 1,2-dithiacyclopentene and 1,2,3-trithiane-5-carboxylic acid :
literature
- Nencki, Marcel (1891). About the occurrence of methyl mercaptan in human urine after asparagus consumption . In: Archives of Experimental Pathology and Pharmacology . 28 (3): 206-209, DOI: 10.1007 / bf01824333 .
- Mechthild Busch-Stockfisch: Lebensmittel-Lexikon , p. 665 ( limited preview in the Google book search).
- RJ Parry, AE Mizusawa, IC Chiu, MV Naidu, M. Ricciardone: Biosynthesis of Sulfur Compounds. Investigations of the Biosynthesis of Asparagusic Acid , Journal of the American Chemical Society 107, No. 8, 1985, pp. 2512-2521, doi : 10.1021 / ja00294a051 .
- R. Singh, GM Whitesides: Comparisons of Rate Constants for Thiolate-Disulfide Interchange in Water and in Polar Aprotic Solvents Using Dynamic 1 H NMR Line Shape Analysis , J. Am. Chem. Soc. 112, No. 3, 1990, pp. 1190-1197, doi : 10.1021 / ja00159a046 .
Web links
Individual evidence
- ↑ Olac Foss, Olav Tjomsland: Crystal and molecular structure of 1,2-dithiolane-4-carboxylic acid , Acta Chemica Scandinavica , 12 (1958) pp. 1810-1818, doi : 10.3891 / acta.chem.scand.12-1810 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b R. Parry, A. Mizusawa, I. Chiu, M. Naidu, M. Ricciardone: Biosynthesis of sulfur compounds. Investigations of the biosynthesis of asparagusic acid. In: Journal of the American Chemical Society . Volume 107, No. 8, 1985, pp. 2512-2521, doi: 10.1021 / ja00294a051 .
- ^ EF Jansen: The Isolation and Identification of 2,2'-Dithiolisobutyric Acid from Asparagus . In: Journal of Biological Chemistry . 176, No. 2, 1948, pp. 657-664. PMID 18889921 .
- ↑ Y. Kitahara, H. Yanagawa, T. Kato, N. Takahashi: Asparagusic acid, a new plant growth inhibitor in etiolated young asparagus shoots. In: Plant and Cell Physiology. Volume 13, No. 5, October 1972, pp. 923-925 doi: 10.1093 / oxfordjournals.pcp.a074805 .
- ↑ Stephen C. Mitchell: Biological Interactions Of Sulfur Compounds . CRC Press, 2004, ISBN 0-203-36252-7 , pp. 161 ( limited preview in Google Book search).
- ↑ ML Pelchat, C. Bykowski, FF Duke, DR Reed: Excretion and perception of a characteristic odor in urine after asparagus ingestion: a psychophysical and genetic study. In: Chemical Senses . Volume 36, No. 1, January 2011, pp. 9-17, doi : 10.1093 / chemse / bjq081 , PMID 20876394 , PMC 3002398 (free full text).
- ^ S. Mitchell: Food Idiosyncrasies: Beetroot and Asparagus . In: Drug Metabolism and Disposition . 29, No. 4 Pt 2, 2001, pp. 539-534. PMID 11259347 .
- ↑ Entry on asparagus. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ^ R. Waring, S. Mitchell, G. Fenwick: The chemical nature of the urinary odor produced by man after asparagus ingestion. In: Xenobiotica. Volume 17, No. 11, November 1987, pp. 1363-1371, PMID 3433805 , doi: 10.3109 / 00498258709047166 .