Atomoxetine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
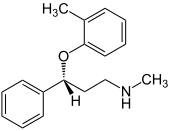
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Atomoxetine | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 255.35 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
172 ° C (hydrochloride) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Atomoxetine is a drug approved for the treatment of attention deficit / hyperactivity disorder (ADHD) . It is marketed under the trade names Atomoxetin , Atomoxe , Agakalin and Strattera (first introduced as Strattera).
Atomoxetine was approved in Germany in March 2005 for the treatment of children and adolescents from the age of 6 and for further treatment of ADHD in adulthood. In June 2013 the approval was extended to the start of treatment in adulthood. In 2017, several generics were approved for the first time in the USA .
It can relieve an associated tic disorder .
The effectiveness of atomoxetine in ADHD is slightly less than that of the stimulants or guanfacine , but it is well documented.
pharmacology
Atomoxetine was originally developed to treat depression , but has proven ineffective for it. The chemical structure is very similar to that of fluoxetine . In contrast to the serotonin reuptake inhibitor (SSRI) fluoxetine, atomoxetine is supposed to selectively inhibit the re-uptake of noradrenaline from the synaptic cleft ( NARI ).
Atomoxetine also acts as an NMDA receptor blocker in clinical doses . This could be relevant for its effectiveness in ADHD, as there is strong evidence that the glutamatergic system is involved in the pathophysiology of ADHD.
Pharmacokinetic data:
- Bioavailability = 63-94%
- Protein binding = 98%
- Plasma half-life = 3.6 hours
Side effects and restrictions on use
In the treatment of children and adolescents, precautionary measures similar to those for serotonin reuptake inhibitors must be observed. Contraindications for use are narrow-angle glaucoma , certain severe cardiovascular or cerebrovascular diseases or the concomitant use of MAOIs .
After the market launch, a significantly increased risk of promoting or triggering aggressive behavior, suicidality and acts of suicide was reported with atomoxetine compared with placebo in children, but not in adults, which must be taken into account when using atomoxetine . There have been very rare spontaneous reports of liver damage manifested as increased liver enzyme levels and increased bilirubin , as well as severe liver damage including acute liver failure. Atomoxetine affects the heart rate and blood pressure, which is why they should be checked regularly.
Trade names
Strattera (D, A, CH, USA), Agakalin (D), Atomoxe (D), various generics (D, USA)
literature
- DB Clemow, C. Bushe, M. Mancini, MH Ossipov, H. Upadhyaya: A review of the efficacy of atomoxetine in the treatment of attention-deficit hyperactivity disorder in children and adult patients with common comorbidities . In: Neuropsychiatric Disease and Treatment . tape 2017 , no. 13 , February 3, 2017, p. 357-371 , doi : 10.2147 / NDT.S115707 .
Web links
Individual evidence
- ↑ a b c data sheet (R) -Tomoxetine hydrochloride from Sigma-Aldrich , accessed on June 16, 2011 ( PDF ).
- ↑ ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9 , p. 162.
- ↑ Press Announcements - FDA approves first generic Strattera for the treatment of ADHD . Retrieved June 2, 2017.
- ^ Opinion on "Attention Deficit / Hyperactivity Disorder (ADHD)". Long version (PDF; 1.0 MB). German Medical Association , August 26, 2005, p. 31.
- ^ AC Childress: A critical appraisal of atomoxetine in the management of ADHD. In: Therapeutics and clinical risk management. Volume 12, 2016, pp. 27-39, doi : 10.2147 / TCRM.S59270 , PMC 4694693 (free full text) (review).
- ↑ AG Ludolph, PT Udvardi, U. Schaz, C. Henes, O. Adolph, HU weigt, JM Fegert, TM Boeckers, KJ Foehr: Atomoxetine acts as at NMDA receptor blocker in clinically relevant Concentrations. In: British journal of pharmacology. Volume 160, number 2, May 2010, pp. 283-291, doi : 10.1111 / j.1476-5381.2010.00707.x . PMID 20423340 . PMC 2874851 (free full text).
- ↑ Ärzteblatt-ADHD: Genetic defect in the glutamate signaling pathway
- ↑ Lesch KP, Merker S, Reif A, Novak M .: Dances with black widow spiders: dysregulation of glutamate signaling enters center stage in ADHD . In: European Neuropsychopharmacology . 23, No. 6, June 2013, pp. 479-491. doi : 10.1016 / j.euroneuro.2012.07.013 . PMID 22939004 .
- ↑ Specialist information Strattera, as of July 2009.
- ↑ Rote-Hand-Brief dated September 29, 2005: on the increased risk of suicide with atomoxetine (PDF; 265 kB).
- ↑ Rote-Hand-Brief on the risk of an increase in blood pressure and heart rate (PDF; 585 kB) retrieved from the website of the Drug Commission of the German Medical Association (AkdÄ).