Barium oxalate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
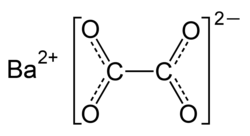
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Barium oxalate | |||||||||||||||
| Molecular formula | BaC 2 O 4 | |||||||||||||||
| Brief description |
white odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 225.35 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.658 g cm −3 |
|||||||||||||||
| Melting point |
400 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Barium oxalate is a chemical compound of barium from the group of oxalates .
Extraction and presentation
Barium oxalate can be obtained by reacting barium chloride with oxalic acid solution or ammonium oxalate.
properties
Barium oxalate and its monohydrate are white, odorless solids that are virtually insoluble in water. The monohydrate converts to anhydrate at 140 to 150 ° C.
The dihydrate has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . The monohydrate and the 3,5-hydrate have a monoclinic crystal structure with the space group C 2 / m (No. 12) and C 2 / c (No. 15), respectively . The hemihydrate and the α-anhydrate have a triclinic crystal structure with the space group P 1 (No. 2) .
use
Barium oxalate monohydrate is used in pyrotechnics and as an analytical reagent.
Individual evidence
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, 2015, ISBN 978-1-4822-6097-7 , pp. 50 ( limited preview in Google Book search).
- ↑ a b c d e f g h data sheet barium oxalate at AlfaAesar, accessed on November 4, 2015 ( PDF )(JavaScript required) .
- ↑ a b P. H. List, L. Hörhammer: Chemicals and Drugs (Am - Ch) . Springer-Verlag, 2013, ISBN 978-3-642-80562-2 , p. 365 ( limited preview in Google Book Search).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entries for barium salts, with the exception of barium sulphate, salts of 1-azo-2-hydroxynaphthalenyl aryl sulphonic acid , and of salts specified elsewhere in this Annex and salts of oxalic acid with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 18, 2017. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ^ Wilhelm Hurka: Chemical internship for physicians . Springer-Verlag, 2013, ISBN 978-3-662-02242-9 , pp. 32 ( limited preview in Google Book search).
- ↑ A. Norl and Christensen, RG Hazell, AMT Bell, A. Altomare: Precision of a crystal structure derived from a synchrotron X-ray powder pattern. The structure of Barium oxalate hydrate, BaC2O4-2H2O. In: Journal of Physics and Chemistry of Solids. 56, 1995, p. 1359, doi : 10.1016 / 0022-3697 (95) 00070-4 .
- ↑ R. Neder, M. Burghammer, H. Schulz, AN Christensen, HG Krane, AMT Bell, AW Hewat, A. Altomare: Crystal structure determination of barium Oxalate, BaC2O4 - 3.5 H2O / D2O. In: Journal of Crystallography - Crystalline Materials. 212, 1997, doi : 10.1524 / zkri.1997.212.4.305 .
- ↑ Huang Sheng-hua, Thomas CW Mak: Refinement of the crystal structure of barium oxalate monohydrate. In: Journal of Crystallography - Crystalline Materials. 190, 1990, doi : 10.1524 / zkri.1990.190.3-4.305 .
- ↑ Axel Nørlund Christensen, Rita Grønbæk Hazell, Ian Charles Madsen: Synthesis and characterization of the barium oxalates BaC2O4-0.5H2O, α-BaC2O4 and β-BaC2O4. In: Acta Crystallographica Section B Structural Science. 58, 2002, p. 808, doi : 10.1107 / S0108768101020717 .
- ^ Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . CRC Press, 2011, ISBN 978-1-4398-1462-8 , pp. 54 ( limited preview in Google Book search).


