Bromoisoval
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
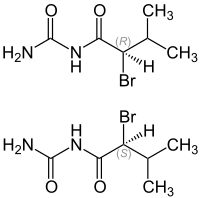
|
||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Bromoisoval | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 11 BrN 2 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 223.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
147-149 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bromisoval is a drug that has been used as a sedative and hypnotic . It was patented by Knoll in 1906 as Bromural ® (out of trade).
chemistry
Chemically, it is, this is a brominated urea - derivative (Bromacylureid) connected to the Carbromal is used. In addition, the bromoacylureides are structurally close to barbituric acid . Bromisoval contains a stereocenter, hence there are two stereoisomers, ( R ) -Bromisoval and ( S ) -Bromisoval. The 1: 1 mixture of ( R ) -Bromisoval and ( S ) -Bromisoval is ( RS ) -Bromisoval, a racemate .
effect
Bromacylureides are less hypnotically effective than barbiturates . In the biotransformation of these agents are bromide - ion free, an elimination half-life comprise of 12 days. This leads to an accumulation with prolonged use .
unwanted effects
If taken for a long time, Bromisoval can lead to chronic bromine poisoning (so-called bromism ) - see Potassium bromide: Usage . Symptoms of an overdose are similar to those of barbiturates , but the mortality rate is very high (4–6%) because of the risk of developing a shock lung . There is also a risk of becoming addicted .
Individual evidence
- ↑ a b J. Falbe, M. Regitz (Ed.): Römpp Lexikon Chemie . 10th edition, Thieme, Stuttgart a. New York, 1996-1999. P. 524.
- ↑ There is not yet a harmonized classification for this substance . A labeling of Bromisoval in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on December 28, 2019, is reproduced from a self-classification by the distributor .
- ↑ a b Entry on Bromisoval in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 28, 2019.
- ↑ a b H. J. Roth, H. Fenner: Medicines . Thieme, Stuttgart a. New York, 1988. p. 272.
- ↑ HPT Ammon (Ed.): Hunnius Pharmaceutical Dictionary . 9th edition, Walter de Gruyter, Berlin a. New York, 2004. p. 258.