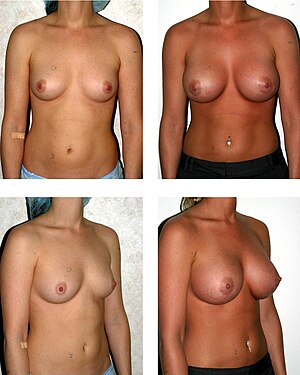
Breast augmentation
The breast augmentation (also Mammaaugmentation ) falls into the field of Plastic and reconstructive surgery and gynecology. The operations are usually carried out for purely aesthetic reasons. If there is a disfiguring malformation of the female breast , it is a medically indicated procedure. This also applies to breast reconstructions after amputation, for example due to cancer (diseases within the meaning of the Social Security Code ).
In Germany, an estimated 15,000 to 20,000 breast augmentations are performed annually, with 30,000 to 45,000 breast implants being sold in Germany every year, according to implant manufacturers. The cost of a breast augmentation is generally between 4,000 and 7,000 euros, with health insurances only reimbursing such services if there is a "medical need". This necessity has to be clarified individually depending on the clinical picture.
The implants used for breast enlargement are medical devices according to the Medical Devices Act .
Due to several serious incidents (burst implants, etc.), these were upgraded to Class III (highest risk class for medical devices) throughout Europe.
The average age of the patients decreases steadily from year to year. Half of the women operated on in 2005 were under 25 years old and 2% under 18 years old. In 2010, 68% were under 25 years old, of which 9% were under 18 years old. At the same time, the average volume increased from 320 cm 3 (ml) to 495 cm 3 , in younger women (under 25 years) from 270 cm 3 to 510 cm 3 .
Breast enlargement should not be confused with breast muscle enlargement in men, where implants lead to a permanent increase in the definition of the breast muscle. However, both operations are similar.
history
Doctors have been involved in rebuilding the female breast since the 1890s. In 1895 the surgeon Vincenz Czerny transplanted a fat tumor, a so-called lipoma , into a woman's breast for the first time . Before that, her real breast was removed because she suffered from breast cancer . Despite the use of the body's own fat, the blood circulation remained inadequate. Experiments with materials such as ivory, bovine cartilage, wool or glass spheres proved to be similarly fatal. Until the late 1950s, all possible uses were tried, such as paraffin injections (by Robert Gersuny ), beeswax or polyethylene , but with no great success. The use of such substances usually led to considerable complications in the form of foreign body reactions such as lipogranulomas . The first fixed implants were used in 1951. Show rupture Ivalon® sponges initially good compatibility, but were immature long term. In 1961, two doctors from Houston ( Texas the first silicone implant on the initiative of Dow Corning Company developed). The first operation took place in 1962. It was launched in 1963. Twenty years later this company was sued by hundreds of women because the controversy had broken out in the USA that silicone implants are the cause of many autoimmune diseases and health problems. In 1992, the Food and Drug Administration (FDA) banned silicone fillings for cosmetic surgery in the United States. After numerous studies and the technical development of the implants, these were allowed again in 2006. They have never been banned in Europe, but there has been a quality seal since 2001 to guarantee the quality for the patients. By 2011, ten million women had been operated on worldwide.
Organized crime uses breast implants to transport drugs.
Surgical technology

During the operation, which is usually performed under general anesthesia , a specialist (specialist in plastic, aesthetic and reconstructive surgery) makes an incision in the skin, lifts the breast tissue and creates an implant pocket into which the breast implant can be inserted. The surgeon then pushes the implant either partially or completely under the pectoral muscle ( submuscular implantation , especially in very thin women with little fat / glandular tissue) or places it under the mammary gland above the pectoral muscle ( subglandular implantation , see picture), whereby the breast tissue itself remains largely unaffected. A third possibility is to place the implant directly inside the muscle stocking and under the fascia layer covering the muscle ( subfascial method , see picture). This method is more time-consuming, more difficult and is only offered by a few surgeons.
The necessary skin incision, of which as little as possible should be seen after the operation, can be in the newly formed underbust crease (infra- mammary access), around or through the areola (transareolar access), in the armpit (transaxillary access) or when using saline solution also take place in the navel. A special technical feature is the endoscopic breast augmentation via the armpit. It was already used in Brazil in the early 1980s by Ivo Pitanguy and has been a routine breast augmentation surgery ever since. A fine endoscopic instrument is placed behind the Chest muscle introduced and this initially blunt and electrocauterically severed in the area of the muscle attachment in order to avoid deformation of the chest when the arm moves. Here, the magnification through the endoscopic image offers greater security, not to cut any sensitive nerves, but to shape the pocket behind the chest muscle with millimeter precision and precision. Both round and anatomical implants up to a size of 480 cm 3 can be safely positioned. In the hands of the experienced surgeon, the technique is just as safe and precise as it is with the incision under or on the breast. The operation time is usually shortened. This technology is not offered by all centers in Germany. There is currently no evidence-based method that is generally preferable. The advantages and disadvantages of the 3 different access routes are v. a. based on the background of the individual requirements and the experience of the respective surgeon with a method and must be discussed in a consultation.
Silicon implants
The basic substance of the shell of the prosthesis today is in almost all cases soft silicone ; This applies in particular to all types of implants with the CE mark - required for sales in the European Union . In one group of breast implants, the silicone shell is coated with polyurethane foam . After experimental evidence that a breakdown product of polyurethane can lead to the formation of sarcomas in rats , the US health authority FDA investigated the carcinogenic effect of the polyurethane foam coating; As a result, in 1995 she put the risk of developing a sarcoma as a result of this at less than 1: 1 million. A distinction is made between smooth and textured versions of the shell structure; The latter grow together with the environment and reduce the likelihood of what is known as capsular contracture due to the disordered alignment of the collagen fiber bundles in the implant capsule . In addition, textured implants are less likely to rotate the implant. The shape of the shell is either symmetrically round / lenticular or "teardrop-shaped" : an asymmetrical shape that is supposed to better replicate the natural shape of the female breast when standing. The advantages of the latter shape are discussed, because it can rotate just like the round shape, but then leads to a clearly visible change in shape. Since it must not twist in the implant position, it is manufactured exclusively with a textured surface.
The shells are filled with physiological saline solution or with silicone gel . The soybean oil , which was propagated at times, was withdrawn from the market after a short time due to insufficient chemical resistance (it became “rancid”). For some time, the silicone filling was suspected of triggering autoimmune diseases or cancer in the event of leakage from a defective shell (which was frequent in earlier decades) . In this respect, silicone implants were banned in the USA in 1996. However, the suspicion could not be confirmed in extensive, worldwide investigations, and in 2006 these implants were approved again in the USA. Newer silicone implants are also manufactured with a more stable shell, which greatly reduces the risk of defects; With the dimensionally stable teardrop-shaped silicone implants, the filling is i. d. Usually chemically cross-linked, so that even in the event of a skin tear it largely retains its shape and does not leak into the surrounding tissue. The consistency of such a silicone cushion is also described as a gummy bear. Round implants sometimes contain even thinner silicone. Implants with silicone gel offer the most natural sense of touch.
The filling with physiological saline solution enables a special procedure in which the implant is only filled during the operation after it has been introduced into the surgical wound. This enables a smaller skin incision and, within certain limits, an individual variation of the volume under visual control. The disadvantage of the saline solution is the possibility of gurgling noises and a less stable shape with occasional wave formation, which can be visible with a thin skin coat. Over the years, these problems can get worse, as there can be a partial loss of the filling (deflation). In a special form as an expander , a valve placed under the skin enables the volume to be changed afterwards. This variant comes from a. used in breast reconstructions to slowly expand the skin.
Autologous fat
In addition to breast enlargement through the implantation of exogenous substances, autologous fat is also used to build up the breast. As part of more recent developments, autologous fat, which is obtained from previously sucked off fatty tissue, is implanted in the breast after the stem cells contained therein have been enriched through a special processing . The use and safety of the method are controversial.
This method, known as Cell-Assisted Lipotransfer (CAL) for breast enlargement , is based on the findings of the Japanese researcher Kotaro Yoshimura from the medical faculty of the University of Tokyo . It reduces the necrosis of larger parts of the implanted cells that has previously occurred with autologous fat transplantation , with the aim of making the treatment result more permanent. The Cellport Clinic Yokohama in Yokohama , Japan, which was the first to offer the treatment worldwide, became famous for this method of breast enlargement . The method is now also used in the USA and Europe. The introduction of the treatment method by an Austrian clinic in July 2007 caused a controversial discussion about the method on Austrian television and the press, which made the method better known.
Another trend coming from the USA has been making its way since 2017. The composite breast augmentation is a combination of the introduction of a silicone implant combined with the introduction of autologous fat.
Medical risks

Essential risk , in addition to the usual surgical risks, is the occurrence of capsular contracture . With every foreign body, the body forms a capsule made of scar tissue ( foreign body reaction ). This capsule can harden the chest in some cases and, in extreme cases, cause deformation and permanent pain. Capsular contractures are the most common complication after the implantation of silicone breast implants. The incidence is around 4 percent after two years and 15 percent after ten years or more.
The better and more professional the procedure, the less likely it is that capsular contracture will occur later , as tissue damage and bleeding increase the risk. There is also a greater risk with a thin skin coat (can then be seen and felt earlier) and with large implants. Surgery that does not go optimally can cause asymmetrical breasts, and sometimes the implants slip, so that reoperations have to be carried out for aesthetic reasons.
The risks of using industrial silicone (without certification for medical purposes) by the company Poly Implant Prothèse (PIP) have not yet been finally clarified. There are reports from the British and French health authorities from 2013.
During a breast augmentation by Carolin Wosnitza , cardiac arrest occurred due to surgical complications, whereupon she died in the following days.
Recovery period
After a breast operation you will be unable to work for about a week and your freedom of movement will be severely restricted. The surgical area is severely swollen and can pull uncomfortably. During this time, doing simple handicrafts like closing the window or pressing a door handle can be painful. Sports activities or physical exertion should generally be avoided for at least six weeks.
In addition to the physical strain to be avoided, water and especially soap should also be avoided. From the 5th day onwards, washing with disinfecting soap solutions is possible again. Full baths are not recommended in the first three postoperative weeks.
To support the breast shape one is taping created. This should be worn for at least five days. It is also recommended to wear a medical bra for at least six weeks, which is provided by the doctors after the operation. Even after this time, physical exertion should only take place to a limited extent. When exercising, it is advisable to wear a tight-fitting sports bra for at least six months.
The breasts themselves will be severely swollen for about 10 to 14 days from the first postoperative day. The final result can only be seen after weeks or months.
Follow-up surgery
According to the FDA , implants are "not a lifelong device". It can be assumed that these will either have to be removed or replaced in a new operation with advancing age. According to manufacturer studies initiated by the FDA, 20 to 40 percent of women with silicone implants have to undergo follow-up surgery within ten years; the rate is higher for implants for breast reconstruction. Reasons for this are ruptures, wrinkles, asymmetries, scarring, pain and infections.
Tumor aftercare
The risk of cancer in women with breast implants is no higher than the average for the population. However, breast prostheses do not interfere with the diagnosis with mammography and sonography . In the case of small breasts, breast augmentation can simplify diagnosis, but the complication rate increases with radiation therapy after breast prosthesis implantation. In early 2011, the FDA published data from a review of work and information on the safety of silicone implants, indicating that people with implants are more likely to develop a very rare type of tumor (ALCL - anaplastic large-cell T-cell lymphoma). With reference to the fact that the very rare cases that occurred were usually associated with typical symptoms, the treating physicians should consider this option if the symptoms are appropriate. No further special preventive examinations or precautionary measures are derived from these findings.
Traceability
The scandal surrounding the implants of the French company Poly Implant Prothèse triggered a Europe-wide public discussion in 2010 about better quality and monitoring regulations. The manufacturing process of medical implants can in principle be traced via their serial and batch numbers; appropriate data are usually given to the patient. This assumes that the manufacturers record their process data seamlessly and keep it accessible over the long term. The European Parliament recently called for this traceability to be made mandatory.
See also
literature
- Viktoriya Sokolova, Matthias Epple: Breast implants - risks and side effects . In: Chemistry in Our Time . tape 46 , no. 2 , 2012, ISSN 0009-2851 , p. 76-79 , doi : 10.1002 / ciuz.201200584 .
- Christopher J. Coroneos, Jesse C. Selber, Anaeze C. Offodile, Charles E. Butler, Mark W. Clemens: US FDA Breast Implant Postapproval Studies . In: Annals of Surgery . 2018, doi : 10.1097 / SLA.0000000000002990 .
Web links
Individual evidence
- ↑ German Society for Aesthetic Plastic Surgery - press release of February 2, 2012: DGÄPC determines figures for breast augmentation in Germany (PDF; 1.4 MB)
- ↑ a b F.-W. von Hesler: The development of breast implants from the first idea to today. In: Sophien-Journal . 01, 2010, pp. 4-5.
- ↑ TT Alagaratnam and WF Ng: Paraffinomas of the breast: an oriental curiosity. In: Aust NZJ Surg 66, 1996, pp. 138-140. PMID 8639128
- ↑ a b Gesche Wüpper: The business with the wrong breasts. welt.de from January 13, 2012 , accessed on January 13, 2012
- ↑ S. Feiel: A Brief History of Breast Operations . 2008.
- ↑ Drug courier from Honduras: woman smuggles 1.5 kilograms of cocaine into breast implants. In: Spiegel Online . June 20, 2015, accessed June 9, 2018 .
- ^ Marita Eisenmann-Klei: Mamma Augmentation. In: Alfred Berger and Robert Hierner (eds.): Plastic surgery. Vol. 3: Mamma trunk genitalia. Springer, Berlin and Heidelberg 2007, ISBN 978-3-540-00143-0 , pp. 155-174, here p. 162.
- ^ Marita Eisenmann-Klei: Mamma Augmentation. In: Alfred Berger and Robert Hierner (eds.): Plastic surgery. Vol. 3: Mamma trunk genitalia. Springer, Berlin and Heidelberg 2007, ISBN 978-3-540-00143-0 , pp. 155–174, here p. 163.
- ↑ Kotaro Yoshimura et al. a .: Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem / Stromal Cells . In: Aesthetic Plastic Surgery . 32, No. 1, 2007, pp. 48-55. 0364-216X.
- ↑ N. Handel u. a .: A long-term study of outcomes, complications, and patient satisfaction with breast implants. In: Plast Reconstr Surg 117, 2006, pp. 757-767. PMID 16525261
- ↑ TF Henriksen et al. a .: Incidence and severity of short-term somlications after breast augmentation: results from a nationwide breast implant registry. In: Ann Plast Surg 51, 2003, pp. 531-539. PMID 14646643
- ↑ I. Kumala et al. a .: Local complications after cosmetic breast implant surgery in Finland. In: Ann Plast Surg. 53, 2004, pp. 413-419. PMID 15502454
- ↑ P. Schmidt-Rhode a. a .: Safety of silicone breast implants. German Society for Gynecology and Obstetrics V .; Guidelines, recommendations, opinions; As of August 2008
- ↑ http://orf.at/stories/2209804/2177316/ Chronology of the scandal - surgeons kicked off avalanche, ORF.at from April 17, 2013, accessed on December 10, 2013
- ↑ Death from megalomania. At: Süddeutsche Zeitung of January 28, 2013 (accessed January 2, 2014)
- ↑ Death after breast surgery. At: Die Welt vom January 21, 2011 (accessed on January 2, 2014)
- ↑ After breast surgery - "Sexy Cora" is dead. In: Focus from January 20, 2011 (accessed on January 2, 2014)
- ↑ The breast augmentation. The time after the operation. In: klinik-am-rosental.de. Retrieved June 20, 2013 .
- ↑ Breast augmentation: silicone implants have a limited shelf life ( memento of September 7, 2012 in the Internet Archive ) aerzteblatt, June 23, 2011
- ↑ B. Herberger: Safety of Breast Implants. In: News health and aesthetics.
- ↑ Spiegel online, April 3, 2014: Medical devices: EU Parliament calls for stricter rules.