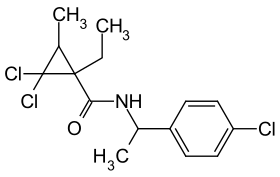
Carpropamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula without stereoisomerism | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Carpropamide | |||||||||||||||
| other names |
2,2-dichloro- N - [1- (4-chlorophenyl) ethyl] -1-ethyl-3-methylcyclopropanecarboxamide |
|||||||||||||||
| Molecular formula | C 15 H 18 Cl 3 NO | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 334.67 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.17 g cm −3 |
|||||||||||||||
| Melting point |
159.7 ° C |
|||||||||||||||
| Vapor pressure |
0.0025 mPa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (0.0018 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Carpropamide is a complex mixture of different isomeric chemical compounds from the group of cyclopropanecarboxamides.
Stereochemistry
Carpropamid of technical quality is a mixture of substances
- (1 R , 3 S ) -2,2-dichloro- N - [( R ) -1- (4-chlorophenyl) ethyl] -1-ethyl-3-methylcyclopropanecarboxamide,
- (1 S , 3 R ) -2,2-dichloro- N - [( R ) -1- (4-chlorophenyl) ethyl] -1-ethyl-3-methylcyclopropanecarboxamide,
- (1 S , 3 R ) -2,2-dichloro- N - [( S ) -1- (4-chlorophenyl) ethyl] -1-ethyl-3-methylcyclopropanecarboxamide and
- (1 R , 3 S ) -2,2-dichloro- N - [( S ) -1- (4-chlorophenyl) ethyl] -1-ethyl-3-methylcyclopropanecarboxamide.
The proportion of the first two stereoisomers mentioned is at least 95%.
effect
Carpropamide is mainly used as a fungicide against rice blight ( Magnaporthe oryzae ). The effect is based on the inhibition of scytalon dehydratase in melanin biosynthesis .
In addition, carpropamide acts as a resistance inducer, which stimulates the induced lignification and the production of the phytoalexin momilactone A.
Admission
With regard to the active ingredient carpropamide, there has never been a regulation in the EU for its use in plant protection products, so its use is not permitted. In Germany, Austria and Switzerland - also because of the lack of rice cultivation - no pesticides with this active ingredient are permitted.
See also
Individual evidence
- ↑ a b c data sheet carpropamide from Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ a b c d e Entry on carpropamide in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on October 9, 2013.
- ↑ a b M. Thieron, R. Pontzen, Y. Kurahashi: Carpropamid: a rice fungicide with two modes of action . In: Plant Protection News Bayer . tape 51 , no. 3 , 1998, p. 257-278 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on carpropamide in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 18, 2016.

