Fenoxanil
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula without representation of the stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fenoxanil | ||||||||||||||||||
| other names |
N - (1-cyano-1,2-dimethylpropyl) -2- (2,4-dichlorophenoxy) propionamide |
||||||||||||||||||
| Molecular formula | C 15 H 18 Cl 2 N 2 O 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 329.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.22 g cm −3 |
||||||||||||||||||
| Melting point |
69-72.5 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water (30 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fenoxanil is a mixture of numerous stereoisomeric chemical compounds from the group of propionamides and a fungicide introduced by BASF and Nihon Nohyaku around 2000 .
use
Fenoxanil is a protective fungicide with systemic effects. It works by inhibiting melanin biosynthesis against rice blotch fungus ( Magnaporthe oryzae ). The commercial product consists of a mixture of four isomers: 85% ( R , RS ) and 15% ( S , RS ).
Stereochemistry
Fenoxanil contains two stereocenters . In general, chemical compounds with several stereocenters form up to 2 n stereoisomers. Here n is the number of stereocenters. According to this, there are four stereoisomers of fenoxanil, which have also been confirmed experimentally:
| Stereoisomers of fenoxanil | |
|---|---|
 CAS number: 143784-54-7 |
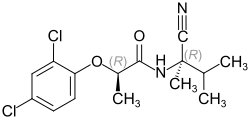 CAS number: 143784-51-4 |
 CAS number: 143784-52-5 |
 CAS number: 143784-53-6 |
Admission
Fenoxanil is not approved as a crop protection agent in the European Union or Switzerland .
See also
Individual evidence
- ↑ a b c d e f g Entry on Fenoxanil. In: Römpp Online . Georg Thieme Verlag, accessed on January 6, 2015.
- ↑ a b c d data sheet fenoxanil from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Paula Y. Bruice: Organic Chemistry: Study compact . Pearson Studium, Munich 2011, ISBN 978-3-86894-102-9 , p. 205.
- ↑ Elim M. Ulrich, Candice M. Morrison, Michael M. Goldsmith, William T. Foremann: Chiral Pesticides: Identification, Description, and Environmental Implications . In: Reviews of Environmental Contaminations and Toxicology. Springer 2012, Boston, Volume 217, pp. 1–74, DOI: 10.1007 / 978-1-4014-2329-4_1 , see p. 10.
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national directory of plant protection products in Switzerland ; Retrieved June 25, 2016.