Cefapirin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
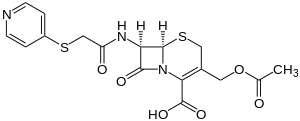
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cefapirin | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Disturbance of cell wall synthesis |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 423.46 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cefapirin is an antibiotic that is used to treat infections caused by susceptible bacteria . It is produced semisynthetically and belongs to the class of cephalosporins of the 1st generation.
In Germany, cefapirin is only approved for use in animals; it is not used in human medicine.
indication
Cefapirine has a broad spectrum of activity against gram-positive and gram-negative organisms. Cefapirin, like most cephalosporins, is therefore effective in treating many infections such as otitis media or urinary tract infections. Compared to beta-lactamases is Cefapirin resistant than penicillins . It can therefore be used against staphylococcal infections.
In veterinary medicine, cefapirin is used to treat or prevent mastitis in cows, and also to treat subacute and chronic endometritis in cows .
Working principle
The cefapirin molecules bind to specific penicillin- binding proteins , which are located in the bacterial cell wall. This prevents further synthesis of the bacterial cell wall.
Application
Cefapirin is administered intramammary (ie through the teat canal into the mammary gland) or intrauterine .
Chemical-pharmaceutical information
The water-soluble cefapirine sodium and cefapirine benzathine (2: 1) are used in pharmaceuticals.
Cefapirin should be stored dry and at room temperature so it can be used for 24 months. A reconstituted solution can be kept at room temperature for 12 hours and in the refrigerator for 10 days. Color changes can occur, but these do not indicate a loss of effectiveness.
Side effects
The most common side effects are hypersensitivity reactions and changes in liver function. However, signs of white blood cell disorder and anemia could also be seen.
synthesis
The synthesis of cefapirine takes place via a semisynthesis , which in this case means the acylation of an amino group . The 7-aminocephalospranic acid (7-ACA for short) to be acylated is converted from penicillin G with the help of N , N ′ -bis (trimethylsilyl) urea through a chemical reaction . The 7-ACA is then acylated in a dehydrochlorinating environment.
Trade names (veterinary medicine)
- Monopreparations
Masti-Safe (D), Metricure (D)
- Combination preparations
with prednisolone : Mastiplan LC (D)
Individual evidence
- ↑ a b Data sheet cefapirin sodium from Sigma-Aldrich , accessed on May 21, 2019 ( PDF ).
- ↑ a b c d e Entry on cefapirine in the DrugBank of the University of Alberta , accessed on May 22, 2019.
- ↑ Metricure available again. In: topagrar online. July 9, 2018, accessed May 20, 2019 .
- ↑ Gunter Schmidt: Cephalosporins . In: Chemistry in Our Time . tape 10 , no. 6 , 1976, p. 189-195 , doi : 10.1002 / ciuz.19760100605 .
- ↑ RM Stockler, DE Morin, RK Lantz, WL Hurley, PD Constable: Effect of milk fraction on concentrations of cephapirin and desacetylcephapirin in bovine milk after intramammary infusion of cephapirin sodium . In: Journal of Veterinary Pharmacology and Therapeutics . tape 32 , no. 4 , 2009, p. 345-352 , doi : 10.1111 / j.1365-2885.2008.01048.x .
- ↑ Intervet Deutschland GmbH: Specialist information Mastisafe . As of March 2016.
- ↑ Intervet Deutschland GmbH: Specialist information Metricure . As of November 2012.
- ↑ European Pharmacopoeia Commission (ed.): European Pharmacopoeia, 9th edition, basic work 2017. Monograph: Cefapirin sodium .
- ↑ External identifiers of or database links for cefapirine sodium, IUPAC name: sodium [(6R, 7R) -3 - [(acetyloxy) methyl] -8-oxo-7 - [[[(pyridin-4-yl ) sulfanyl] acetyl] amino] -5-thia-1-azabicyclo [4.2.0] oct-2-en-2-carboxylate] : CAS number: 24356-60-3, EC number: 246-194-2 , ECHA InfoCard: 100.041.980 , PubChem : 23675312 , ChemSpider : 390127 , DrugBank : DB01139 , Wikidata : Q27106128 .
- ↑ External identifiers or database links for cefapirine benzathine : CAS number: 97468-37-6, EC number: 619-270-1 , ECHA InfoCard: 100.108.326 , PubChem : 167441 , ChemSpider : 146482 , Wikidata : Q27271322 .
- ↑ Entry on cefapirin at Vetpharm, accessed on May 20, 2019.
- ↑ Alle Bruggink (Ed.): Synthesis of β-Lactam Antibiotics: Chemistry, Biocatalysis & Process Integration. Springer Science + Business Media, ISBN 978-0-7923-7060-4 , p. 15 .
- ↑ Entry on CEPHAPIRIN in the Hazardous Substances Data Bank , accessed on May 27, 2019.