Cerium (III) nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
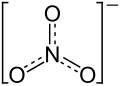
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cerium (III) nitrate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 434.22 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.38 g cm −3 |
|||||||||||||||
| Melting point |
150 ° C |
|||||||||||||||
| boiling point |
200 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cerium (III) nitrate is an inorganic chemical compound of cerium from the group of nitrates , which is usually present as hexahydrate.
properties
As a hexahydrate, cerium (III) nitrate is a solid in the form of colorless tablets or very small prisms. It has a triclinic crystal structure with space group P 1 (space group no. 2) . At 100 ° C it converts into the trihydrate.
use
Cerium (III) nitrate can be used as a catalyst . It also serves to separate cerium from other rare earths. It was previously used in combination with thorium nitrate for mantles .
Individual evidence
- ↑ a b c d R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 372 ( limited preview in Google Book search).
- ↑ a b c d e f data sheet Cerium (III) nitrate hexahydrate, 99.999% trace metals basis from Sigma-Aldrich , accessed on October 30, 2016 ( PDF ).
- ↑ Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , pp. 6 ( limited preview in Google Book search).
- ↑ a b data sheet Cerium (III) nitrate hexahydrate, REacton®, 99.99% (REO) from AlfaAesar, accessed on November 2, 2016 ( PDF )(JavaScript required) .
- ↑ M. Adib, K. Ghanbary, M. Mostofi, MR Ganjali: Efficient Ce (NO 3 ) 3 × 6H 2 O-catalyzed solvent-free synthesis of 3,4-dihydropyrimidin-2 (1H) -ones. In: Molecules . 11, 2006, pp. 649-654, PMID 17971737 .
- ↑ Georg Frerichs, Georg Arends, Heinrich Zörnig: Hager's handbook of pharmaceutical practice, first volume for pharmacists, drug manufacturers, druggists, doctors and medical officials . Springer-Verlag, 2013, ISBN 978-3-642-90728-9 , p. 907 ( limited preview in Google Book search).


