Chloroxuron
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
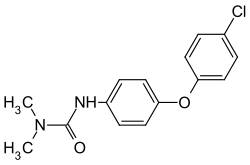
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chloroxuron | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 15 H 15 ClN 2 O 2 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 290.75 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.34 g cm −3 |
|||||||||||||||
| Melting point |
151 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (4 mg l −1 ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chloroxuron is a chemical compound from the group of phenylureas .
Extraction and presentation
Chloroxuron can be obtained through a multi-stage reaction: First, p -chlorophenol is reacted with p -chloroaniline and potassium hydroxide ; the intermediate product 4-chloro-4-phenoxyaniline formed gives chloroxuron with phosgene and subsequent reaction with dimethylamine .
properties
Chloroxuron is a flammable, colorless, odorless, non-volatile solid that is practically insoluble in water. It decomposes when heated. It degrades relatively quickly in the soil.
use
Chloroxuron is mainly used as a herbicide in vegetable growing. It was developed by Ciba-Geigy in 1960 and was approved in the GDR between 1970 and 1994 and in the FRG between 1971 and 1990.
In 2002, the EU Commission decided not to include chloroxuron in the list of permitted active ingredients in pesticides.
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e f g h i j Entry for CAS no. 1982-47-4 in the GESTIS substance database of the IFA , accessed on April 24, 2016(JavaScript required) .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 221 ( limited preview in Google Book search).
- ↑ a b M. Bahadir, H. Parlar, Michael Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 978-3-540-63561-1 , pp. 265 ( limited preview in Google Book search).
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 , pp. 480 ( limited preview in Google Book Search).
- ↑ Peter Brandt (Ed.): Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products ; Approval history and regulations of the Plant Protection Application Ordinance . Springer, 2010, ISBN 978-3-0348-0028-0 , pp. 12 ( limited preview in Google Book search).
- ↑ Regulation (EC) No. 2076/2002 of the Commission of November 20, 2002 (PDF) extending the deadline according to Article 8 (2) of Council Directive 91/414 / EEC and on the non-inclusion of certain active substances in Annex I of this Directive and the revocation of the approval of plant protection products with these active substances.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on chloroxuron in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved February 19, 2016.


