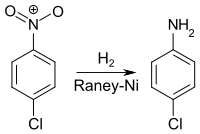
4-chloroaniline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-chloroaniline | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 6 ClN | ||||||||||||||||||
| Brief description |
beige solid with a slightly aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 127.57 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.43 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
70 ° C |
||||||||||||||||||
| boiling point |
232 ° C |
||||||||||||||||||
| solubility |
2.2 g l −1 at 25 ° C in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.06 ml m −3 , 0.3 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-chloroaniline is an organic-chemical compound from the group of chloroanilines , which is used as an intermediate in the manufacture of pesticides , isocyanates , dyes and pharmaceuticals .
synthesis
4-chloroaniline are obtained by hydrogenation of 4-chloronitrobenzene with Raney nickel as catalyst .
An alternative production route is the chlorination of acetanilide with sodium hypochlorite in the presence of a mineral acid and subsequent hydrolysis .
By electrolytic reduction of nitrobenzene in the presence of hydrochloric acid , a mixture of 2- and 4-chloroaniline, which can be separated via the corresponding acetanilides.
use
4-chloroaniline can be used as a diazo component for the production of azo dyes. Since 4-chloroaniline can be released again from these dyes under reductive conditions, according to the Consumer Goods Ordinance (BedGgstV) they may not be used for textile and leather products that may come into direct contact with human skin or the oral cavity for a longer period of time (e. B. clothing, bed linen, towels, shoes, gloves, yarns and fabrics intended for the end user).
4-chloroaniline is used in the manufacture of the herbicide monolinuron and the drugs flunitrazepam and demoxepam .
toxicology
4-chloroaniline is similar to aniline in terms of its toxic effect , but has a higher lipophilicity , so that it is more easily absorbed into the cells. The acute toxic effect of the compound is based on the strong binding affinity for hemoglobin . Studies of the carcinogenic effects of 4-chloroaniline in humans are not available, but animal studies suggest that it is carcinogenic.
Individual evidence
- ↑ a b c d e f g h i j k Entry on 4-chloroaniline in the GESTIS substance database of the IFA , accessed on August 3, 2020(JavaScript required) .
- ↑ Entry on 4-chloroaniline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 3, 2020. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Klaus Schwetlick et al: Organikum . 21st edition. Wiley-VCH, Weinheim 2001, ISBN 3-527-29985-8 , pp. 627 f .
- ↑ EN Abrahart: Dyes and Their Intermediates . 2nd Edition. Edward Arnold Ltd., London 1977, ISBN 0-7131-2580-2 , pp. 40 .
- ↑ Walther Löb: Electrolytic reduction of nitrobenzene in hydrochloric acid . In: Reports of the German Chemical Society . tape 29 , no. 2 , May 1896, p. 1894 , doi : 10.1002 / cber.189602902150 .
- ↑ Consumer Goods Ordinance, Appendix 1 (to Section 3). Substances that may not be used in the manufacture or treatment of certain commodities. Federal Ministry of Justice and Consumer Protection, accessed on August 3, 2020 .
- ↑ Justification for p-chloroaniline. (PDF) Federal Institute for Occupational Safety and Health, March 21, 2019, accessed on August 3, 2020 .