Chlorophacinone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
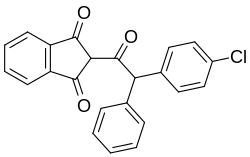
|
||||||||||||||||
| Racemate | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Chlorophacinone | |||||||||||||||
| other names |
( RS ) -2- (α- (4-chlorophenyl) phenylacetyl) indan-1,3-dione |
|||||||||||||||
| Molecular formula | C 23 H 15 ClO 3 | |||||||||||||||
| Brief description |
light yellow odorless crystal needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 374.82 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
approx. 0.6 g cm −3 |
|||||||||||||||
| Melting point |
138 ° C |
|||||||||||||||
| boiling point |
240 ° C at 0.8 mbar |
|||||||||||||||
| solubility |
practically insoluble in water (3.4 mg l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chlorophacinone is a chemical compound from the group of indane derivatives (more precisely from 1,3-indanedione ) and organic chlorine compounds .
Extraction and presentation
Chlorophacinone is produced by various synthetic routes. A frequently used process starts from phenylacetone , which is reacted with bromine in chlorobenzene to form 1-bromo-1-phenylacetone . This then reacts with chlorobenzene in the presence of aluminum trichloride under anhydrous conditions to form 1- (4-chlorophenyl) -1-phenylacetone, which then with dimethyl phthalate in the presence of sodium methoxide finally gives the target compound chlorophacinone .
properties
Chlorophacinone is a flammable light yellow solid which is practically insoluble in water. It decomposes at temperatures above 320 ° C. Rapid photolysis occurs outdoors . In the soil, the breakdown takes place mainly through ring opening to 4-chlorodiphenylacetic acid .
use
Chlorophacinone is used as a rodenticide against field voles , ground voles and bank voles . It works by inhibiting blood clotting by blocking prothrombin formation .
Admission
In Germany, the approval was revoked by the Federal Office for Consumer Protection and Food Safety on June 30, 2010. In the EU states such as Germany and Austria as well as in Switzerland, no pesticides are permitted that contain chlorophacinone as an active ingredient.
Individual evidence
- ↑ a b c d e f g h i j Entry on chlorophacinone in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on Chlorophacinone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Patent DE102005055528 : Process for the production of 2 - [(4-chlorophenyl) -phenylacetyl] -1,3-indandione (chlorophacinone). Registered on November 22, 2005 , published on April 10, 2008 , applicant: CPI ChemiePark Institute, Bitterfeld, inventor: Alexander Barthel, René Csuk.
- ↑ a b M. Bahadir, H. Parlar, Michael Spiteller: Springer Umweltlexikon . 2000, ISBN 978-3-540-63561-1 ( page 266 in the Google book search).
- ↑ BVL - Technical reports - Revoke approvals of plant protection products with the active ingredients Brodifacoum, chlorophacinone and lecithin. Retrieved June 3, 2019 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Chlorophacinone in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved March 3, 2016.


