Phenylacetone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phenylacetone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 10 O | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 134.18 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.01 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−15 ° C |
||||||||||||||||||
| boiling point |
216 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5165 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Phenylacetone is a colorless to slightly yellow liquid with a strong, characteristic odor that is used in the chemical and pharmaceutical industries.
presentation
Phenylacetone ( 4 ) can be prepared in a wide variety of ways. The most economical is the Dakin-West reaction of phenylacetic acid ( 1 ) and acetic anhydride ( 2 ) with base catalysis. The enolate of the mixed anhydride attacks another molecule 2 , splits off acetate and the resulting β-keto acid 3 decarboxylates to 4 .
Another method (II) uses the acetates of divalent metals, especially lead , as the source of acetyl .
The reaction of methyllithium with the lithium salt of phenylacetic acid represents a further elegant route to 4 , even if half of the MeLi has to be used for the deprotonation of 1 .
Further syntheses that manage without the monitored substance phenylacetic acid are possible, but due to the price or the toxicity of individual starting materials or due to low yields, they can only be carried out on a laboratory scale. I.a. it is possible to react benzene with chloroacetone in a Friedel-Crafts alkylation (a) or to combine acetone itself with benzene in a radical manner (b); the manganese (III) acetate required for this is the limiting reagent.
Ketones can be prepared by the reaction of organometallic reagents with nitriles : In our case, this means that either trimethylaluminum (or methylmagnesium halide) can be allowed to act on benzyl cyanide (a) or, on the other hand, benzyl magnesium bromide can act on acetonitrile (b); in both cases hydrolysis of the intermediate imine gives ketone 4 .
The oxidation of α-methylstyrene with thallium (III) salts in methanol (VI) is also of interest : After the addition to the double bond, Tl (I) is split off and a sigmatropic rearrangement occurs at the intermediate carbocation . The resulting dimethyl ketal is then acid catalysis hydrolyzed .
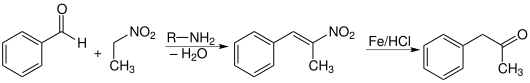
Another way is the condensation of benzaldehyde and nitroethane , which gives phenyl-2-nitropropene , which can be reduced to phenylacetone via an oxime as an intermediate.
use
Phenylacetone is used for the synthesis of pesticides and pharmaceuticals . Examples are diphacinone (a rat poison ) or amphetamine (by reductive amination ). Due to their potential use in the synthesis of various amphetamines, both manufacture and sale require a permit.
Legal
Due to its suitability as a starting material in methamphetamine synthesis , phenylacetone belongs to category I of the monitored chemicals in the EU and Switzerland according to the Basic Substance Monitoring Act . This means that manufacturing, trading and importing and exporting without a permit are punishable by law.
Individual evidence
- ↑ a b c Entry on 1-phenylpropan-2-one. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e Entry on phenylacetone in the GESTIS substance database of the IFA , accessed on May 10, 2018(JavaScript required) .