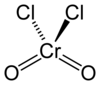
Chromium (VI) oxide dichloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chromium (VI) oxide dichloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CrO 2 Cl 2 | ||||||||||||||||||
| Brief description |
volatile, blood-red liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 154.90 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.91 g cm −3 |
||||||||||||||||||
| Melting point |
−96.5 ° C |
||||||||||||||||||
| boiling point |
117 ° C |
||||||||||||||||||
| Vapor pressure |
26.6 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
violent decomposition in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chromium (VI) oxide dichloride is a chemical compound from the group of metal oxides or chlorine compounds . It is in the form of a volatile, blood-red liquid that smokes in moist air.
Extraction and presentation
Chromium (VI) oxide dichloride can be produced by reacting hydrogen chloride with chromic acid or chromium trioxide .
It can also be produced by reacting potassium chromate with sodium chloride and sulfuric acid.
properties
Chromium (VI) oxide dichloride is highly electrophilic and has an oxidizing effect. It decomposes violently in water, producing hydrochloric acid or chlorine vapors as well as chromic acid . The compound's vapors are five times as heavy as air.
use
Chromium (VI) oxide dichloride is used as an oxidizing agent (e.g. in the conversion of alkenes to aldehydes ), in the tannery as a pickling agent and as a detection agent for chlorine.
safety instructions
Chromium (VI) oxide dichloride is classified as carcinogenic and mutagenic category 2. It is not flammable, but strongly oxidizing.
Web links
Individual evidence
- ↑ a b c d e f g h Entry on chromium (VI) oxide dichloride in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on Chromyl dichloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 .







