Cobalt hydroxide oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
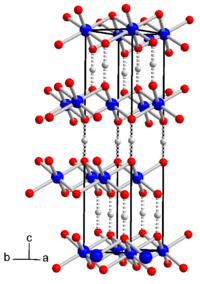
| Crystal structure of cobalt hydroxide oxide. Dashed lines: hydrogen bonds. __ Co 3+ __ O - __ H + |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cobalt hydroxide oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | CoO (OH) | |||||||||||||||
| Brief description |
brown solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 91.93 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.29-4.90 g cm -3 |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cobalt hydroxide oxide is an inorganic chemical compound from the group of hydroxides .
Extraction and presentation
Cobalt hydroxide oxide can be obtained by reacting cobalt (II) hydroxide with oxygen .
properties
Cobalt hydroxide oxide is a brown powder. At over 150 ° C the compound is increasingly decomposed with cobalt (II, III) oxide formation and the simultaneous release of oxygen and water. It is soluble in hydrochloric acid with evolution of chlorine and sparingly soluble in nitric acid and sulfuric acid . Organic acids such as oxalic acid and tartaric acid dissolve it under reduction. Alkaline solutions and ammonia do not attack it. The compound has a trigonal crystal structure with the space group R 3 m (space group no. 166) and the lattice parameters a = 285.5 pm and c = 1315.7 pm). It consists of layers of AlO 6 octahedra that are connected by hydrogen bonds. A hexagonal shape is also known in the space group P 6 3 / mmc (No. 194) .
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1666.
- ^ Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . Taylor & Francis US, 2011, ISBN 1-4398-1462-7 , pp. 138 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ M. Deliens, H. Goethals: Polytypism of heterogenite. In: Mineralogical Magazine , 39, 1973, pp. 152-157, PDF .
