Creophilus maxillosus
| Creophilus maxillosus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Creophilus maxillosus on dead wild boar with fly and insect larvae |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Creophilus maxillosus | ||||||||||||
| ( Linnaeus , 1758) |
Creophilus maxillosus is a beetle from the family of Rove . The genus Creophilus is onlyrepresentedin Europe with this species . There are currently 16 species worldwide. Creophilus maxillosus occurs in Europe in the subspecies Creophilus maxillosus maxillosus . The subspecies Creophilus maxillosus villosus , whichoccurs mainly in North America,was previously listed as a separate species Creophilus villosus .
The beetle is used in forensic entomology to estimate the time interval that has passed after death.
Comment on the name
It is not surprising that the conspicuous and common species is already listed in 1758 in the tenth edition of Linné's Systema Naturae , in which the binary nomenclature commonly used today is used for the first time . Linnaeus describes the species under the name Staphylinus maxillosus . He gives four older sources, for example the beetle was already described by Linnaeus in 1746 as Staphylinus ater glaber, maxillis longitudine capitis (Latin: black hairless staphylinus with maxillae the length of the head ). The part of the name maxillosus thus refers to the large maxillae (shaded red in Fig. 4). However, this property was at most helpful for differentiating a few species; with increasing knowledge of species, the maxilla length is no longer of diagnostic value. Since Linnaeus's statement about the length of the head is not obvious about the maxilla , and on the other hand the mandibles (Fig. 9) are significantly longer than the maxilla, an English book translates maxillosus undifferentiated as 'large-jawed' and indirectly expresses the assumption that Linnaeus didn't mean the lower jaw, but the upper jaw.
In the volume Der Käfersammler der Illustrierte Taschenbücher für die Jugend ( The Beetle ) , the beetle is shown under the German name 'Weisshaar-Halbflügler' because of the light band on the strongly shortened wing covers . However, this name did not catch on. In a modern non-fiction book, the beetle is called 'Carrion Beetle'.
The name Staphylinus can be found in Muffets Theatrum Insectorum as early as 1634 . Muffet distinguishes between two different staphylinus . In the ninth edition of the Systema Naturae from 1756, Linné also distinguishes only two staphylinus . In the tenth edition two years later, however, Linnaeus already separates the genus Staphilinus into 19 species. Leach is considered to be the author of the genus Creophilus in 1819. More precisely, it was Samouelle who published in 1819 that he was informed by Leach that Kirby had now separated the genus Creophilus with the type Creophilus maxillosus from the genus Staphylinus . Because of this, the author of the genre is occasionally given as Samouelle or Kirby.
The name Creōphilus is derived from the ancient Greek κρέας kréas 'meat' and φίλος phílos 'friend' and alludes to the fact that the animal is found on rotting meat, especially on wheat ( taxo picture ).
features
The fifteen to twenty-five millimeter long beetle can hardly be confused. The head and pronotum are shiny black, the head is only hairy on the temples, the pronotum is only hairy on the lateral edge (Fig. 6). A vaguely delimited, wide, jagged cross-band of gray hair with black-haired spots runs over the wing covers, which leave most of the abdomen free. The abdomen is hairy piebald whitish or yellowish gray on the upper side, the underside of the abdomen is dense, predominantly lightly haired in Creophilus maxillosus maxillosus (Fig. 2), in Creophilus maxillosus villosus only lightly haired on the front, densely dark on the back (Fig. 3). Different variants have been described, sometimes as subspecies or distinct species ( anonymus Sulzer, arcticus Erichson, balteatus De Geer, bicinctus Mannerheim, canariensis Bernhauer, ciliary Stephens, ciliaroides Hatch, cinearius Erichson, fasciatus Laporte de Castelnau, fulvago Motschulsky, imbecillus , Sharp medialis Sharp, nebulosus Geoffroy, orientalis Motschulsky, pulchellus Meier, sikkimensis Wendeler, subfasciatis Sharp). The more precise distinction between the two subspecies can be found in Richardson , a key for some of the variants in Fauvel .
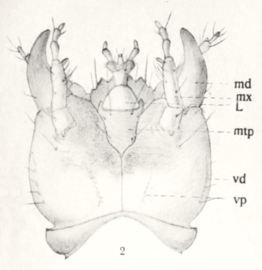
The head is neck-shaped at the back (Fig. 6). In females and weaker males, the head is rounded and widens towards the rear (Fig. 2), in strong males it is as wide or even wider in front of the eyes than in front of the neck (Fig. 1). The head is punctured moderately densely above . The large oval eyes are far apart on the top of the head. They are almost flat and slightly sloping, further apart in front than behind. The cord-shaped antennae are black and a little longer than the head. They are eleven-limbed, the last five limbs form an elongated club, the limbs of which are wider than they are long. The first six links are shiny and covered only with individual protruding hairs, the last five links are densely pubescent and therefore dull. The end link is significantly longer than either of the two preceding ones and ends in a concave cutout (only visible with the appropriate orientation, as in Fig. 7). The upper lip is broad and bilobed (clearly visible in Fig. 1 when fully enlarged). The left upper jaw has two small, roughly equal-sized teeth in the middle of the inner edge, which are separated by a deep notch. The right upper jaw shows three even smaller teeth. Two are at the level of the cutting edge (outlined in green and blue in Fig. 9), the third is lower and is covered by the upper anterior tooth when viewed from above. It only becomes visible if you look more from the inside (outlined in pink in Fig. 9). The upper tooth (outlined in blue) further behind is significantly more powerful than the other two in strong males. The lower jaws (tinted red in Fig. 4) have thread-like, four-part jaw buttons (tinted green in Fig. 4). The end link is shorter than the third link. The lower lip has a bulged, membranous tongue (white arrowhead in Fig. 4). The leathery side tongues, which lie close to it, have lashes on the inside, protrude clearly beyond the tongue. The three-part lip buttons (tinted blue in Fig. 4) are also thread-shaped.
The pronotum is significantly wider than it is long. The front corners are rounded. The pronotum narrows towards the base and merges into the base without any discernible posterior angles. The margins are very shallow. The sides and base are finely edged. The pronotum is only very finely dotted at the edges. The rear part of the folded sides of the pronotum ( epipleuren ) is turned back so much that it is not visible even from the side. In Fig. 2, only the anterior half of the pronotum shows the epipleuras as narrow, bare stripes.
The elytra are together somewhat wider than the pronotum. They leave the last six of the eight abdomen gites uncovered. The outer angles are strongly rounded and they meet the seam at an obtuse angle. They are densely covered with short hairs of about the same length, from which several hairs that are many times longer protrude.
The hind wings are fully developed and functional. At rest they are folded across several times and stored under the shortened wing covers.
The label is longer than it is wide and heart-shaped and triangular. It is dense and strongly dotted and predominantly hairy gray.
The piebald hair on the upper side of the abdomen is interrupted by a median black median stripe and short, wide horizontal stripes. The abdomen ends rounded with the eighth tergite . The ninth and tenth abdominal segments form the external sexual organs and are developed differently in the two sexes, but show long hairy paired appendages in both sexes, but in dried specimens only their tips are visible (compare taxo image and Fig. 10 with Figs. 1 and 2 ).
The hip , thigh ring and thigh are short with black hair. The rails are long spiky hairy. All splints have two end spikes of unequal length on the underside, the significantly longer one on the inside (Fig. 5). In the front splints, however, the end spines are not very noticeable between a ring of spines. The tarsi are five-part. The tarsi of the forelegs are rounded (Fig. 6) and have a cushion of adhesive hair underneath. In contrast to the genus Staphylinus , the middle hips are broadly separated from each other.
Figure 14 shows the male sexual organ (aedeagus). A more detailed drawing and illustration of the female genitals can be found in Clarke. A very detailed description of the adult beetle with many detailed drawings can be found at Blackwelder .
Stages of development
egg
The egg is two to three millimeters long and 1.5 to two millimeters thick. It is thin-skinned, opaque and white to cream-colored.
Larval stages
The larva was drawn in exact detail by Westwood as early as 1839 (Fig. 11), but the author was not sure whether it was the larva of Goerius olens or Creophilus maxillosus . This uncertainty was decided by Kemner in 1912 to the effect that it was beyond doubt the larva of Creophilus maxillosus . Kemner described the larva again and in more detail.
The newly hatched larva reaches a length of 5.5 to six millimeters and a width of 1.2 to 1.5 millimeters. In the last stage (pictures on the net) it is twenty-five to thirty millimeters long and five to seven millimeters thick. It is described below.
The head is rounded and flat. There are four ocells on each side (Fig. 11, detail 2). The tripartite antennae (Fig. 11, Detail 3) arise far in front, almost between the mandibles (Fig. 11, Detail 4 and Fig. 12, md). They are just under two millimeters long. The structure of the maxilla (Fig. 11, Detail 5 and Fig. 12, Mx) and the lower lip with lip probe (Fig. 11, Detail 6 and Fig. 12, L) can be seen in the illustrations.
The back of the first breast segment is broad, dark and heavily sclerotized. The ventral side is only sclerotized in front. The belly plate is triangular with an appendix. The middle and rear chest are built similarly to one another: their back plates are a little wider than those of the first breast segment, but significantly shorter. They are divided in two lengthways and form a gap of 0.1 millimeters. The ventral side of the second and third breast sections is not sclerotized. In all three back plates of the chest section, a narrow sclerotized strip is set off at the rear.
With the exception of the distal phalanx, all abdominal segments have two clearly separated, adjacent plates on the back, two hexagonal plates lying next to one another on the stomach and two plates on the sides, the upper one larger and elongated, the lower one hardly smaller. All of these eight plates are dull brown and very sparsely hairy. The last abdominal segment (Fig. 11, detail 8) is darker, heavily sclerotized and hairy everywhere. It has two three-part cerci , each about three millimeters long . The middle link is significantly smaller than the other two. The two cerci arise at the end of the back and protrude backwards and upwards. On the abdomen, the last abdominal segment has a two millimeter long tubular extension through which the digestive tract is emptied.
The six legs are all built in the same way (Fig. 11, detail 7). The hips are large, long and broad at the base. The leg ring adjoins the leg at an angle. The leg is one millimeter long and widens towards the rail. The rail of the same length is very narrow. The foot consists only of a strong claw. All parts of the leg are hairy spiky, on the splint the hair is arranged in two rows.
The side of the chest section has a large stigma in the middle and a small stigma between the second and third pair of legs. The abdomen becomes smaller towards the back. All spiracles are darkly sclerotized.
Doll
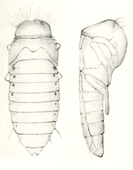
The pupa (Fig. 13) is fifteen millimeters long and five millimeters wide at the widest point. It is initially translucent light brown and then turns black. Legs, hind wings and jaws are folded against the abdomen. The antennae cross the upper jaws under the eyes. Behind it, they are converging and then bent backwards. The wing covers lie over the hind wings and curve back and down to the body, but only partially cover the hind wings. The abdomen is flat on top and sharply edged on the sides. On top of it, the dark stigmas lie conspicuously elevated on the outside of the abdominal segments (picture in the network). A conspicuous, narrow ridge runs across the front part of the pronotum, on which a row of individual hairs sits at equal intervals (clearly visible in Fig. 13 when enlarged).
biology
Occurrence
The beetles are mainly found on carrion that is in open terrain. But carrion is also served in wooded areas. Carcasses lying in damp places are preferred to those in dry places, as are larger carcasses over small ones. The beetles are also often found on dung heaps. In a study in the Caucasus, the beetle was detected at all altitudes from the plain to the nival zone .
The beetle can be found all year round. The two frequency maxima are in early summer on the one hand and in early autumn on the other. The beetle is particularly active at night.
Food, food intake
Considerable distances have to be covered to find the carrion. The carrion is mainly localized in flight, but the smell of nearby carrion also attracts the animals directly from their underground hiding places and the beetles then approach with the help of their legs.
The beetles' main food is fly larvae that develop in the carrion. The carrion of fish, frogs, turtles, snakes, lizards, birds, large and small mammals serves as a substrate for the hunted fly larvae. After periods of starvation in captivity, the beetles react visibly nervous when fly larvae are placed in the cage. The smell therefore plays a role in tracking down the victims. The hungry beetle can then eat up to ten larvae in quick succession. The larva is grasped with the upper jaws, the larval skin is severed with the jaws, and the beetle also holds the larva with the front legs while standing on the middle and rear pair of legs. Then the larva is kneaded with the jaws forwards and backwards until the resulting pulpy mass is absorbed. What remains is the exoskeleton of the larva. In the case of very large larvae, the beetles also like to lie on their side while eating.
When trying to feed in captivity, hungry beetles also eat live ants, termites, earthworms and flightless flies. They attack the prey, hold it with their front legs, crack the prey's exoskeleton with their upper jaws and then eat the exposed soft tissues.
Larvae of their own species are also eaten. The first two larval stages can usually crawl into the upper soil layers when attacked because of their small size. Large larvae in the third instar try to defend themselves against attacking adult beetles with their upper jaws . However, this does not always work. It was observed that two beetles attacked a larva at the same time and ultimately tore it in two. Each attacker then withdrew with his half and ate it. When fighting over prey, adult animals also attack each other, which can damage each other considerably. If the weaker beetle is finally eaten by the stronger in the cage, this may always be due to the fact that the weaker beetle does not find enough alternatives due to the lack of space in the cage.
The carrion itself is only used as a source of food when the beetles are in dire need of food. However, fly larvae are preferred, the presence of flies promptly prompts the beetles to stop taking in carrion. When it comes to choosing its prey, the beetle also differentiates between different types of flies. In a series of experiments it was shown that the beetle ate larvae of the fly species Cochliomyia macellaria over the larvae of Chrysomya rufifacies . For example, only 4.8% of the Cochliomyia macellaria larvae killed by him were not eaten, while almost every fourth fly larva of the Chrysomya rufifacies larvae was spurned, even though it had previously been killed by the beetle. In addition, the less preferred type of maggot, a little over 42%, was not attacked at all, while the maggots of Cochliomyia macellaria only spared 32%. In the study area in which Cochliomya macellaria native, Chrysomya rufifacies contrast, invasive occurs, avoid Creophilus maxillosus in nature carrion, predominantly of Chrysomya rufifacies is populated.
The beetle's larvae feed exclusively on fly larvae.
Use in forensics
The presence of beetles on corpses is used in forensic entomology to narrow down the time of death. The beetle is one of the late species. It is true that individual specimens of the beetle can be seen flying in just a few hours after death, but forensics is investigating when the beetle appears more frequently or when larvae or pupae are found for the first time and how long larvae can be found on a carcass. In a detailed study in which different forest types and different seasons were compared in two years, it was shown that larvae of Creophilus maxillosus on laid out pig carcasses over both years and three forest types averaged on 21-day-old carcasses in spring and on seven in summer Day-old carcasses and appeared on 12-day-old carcasses in the fall. Averaged over the seasons, the larva appeared in the pine-oak forest on 23 to 25-day-old carrion, in the hornbeam-oak forest on 26 to 28-day-old carrion, and in the alder forest on 18 to 20-day-old carrion. However, the results also depend on the year. Averaged over forest types and seasons, the larvae appeared on the carrion after 13 to 15 days in 2006, but only on 18 to 20 day old ears in the following year. In addition to the first occurrence, the length of occurrence was examined. In spring, the larvae can be found for an average of 35 days, in summer, however, only for ten days. In a comparison of undamaged pig carcasses and pig carcasses severely damaged by fire, which was carried out once in summer and once in winter, Creophilus maxillosus only appeared on burned pigs after about four weeks in winter, and earlier on burned pigs in summer than on unburned pigs.
The time interval that elapses before the beetle or its larvae appear on the carcass (preappearance interval PAI, measured in days) can be roughly described by an exponential function of the temperature T in ° C ( ). A specific series of tests yielded the following results: If the shortest time interval after which the beetle or its larva was found on the carcass was used for c, the result for the larva of Creophilus maxillosus was c = 7.3; by regression was obtained for a and b, the values a = 7.432066 and b = 0.312329. For the adult animals, c = 2.4 was obtained for the parameters a = 8.149812 and b = 0.377601. If all three parameters a, b, and c were determined by regression, a larger c was obtained in both cases. A further investigation shows that in the case of Creophilus maxillosus the time span since death under known temperature conditions can also be better estimated from the size of the hatched finished insect, even more precisely if one considers males and females separately. At a given temperature, larger and heavier animals need less time for the individual developmental steps than smaller, lighter animals.
Special behaviors
Thanatose
Both the larva and the beetle can pretend to be dead ( thanatose ). You bend the abdomen downwards and forwards so that the body forms a ring. This posture is only adopted after a sudden disturbance and only for a few seconds. If the triggering stimulus is repeated, thanatose does not occur.
Defensive glands
In particular, the beetle can push the last segments of the abdomen into one another like a telescope. This is made possible because the sclerotized parts are connected by joint membranes. The last tergite above the anus is referred to here as the eighth tergite. (The numbering is not uniform.) The synovium between the seventh and eighth segment (red in Fig. 10) is attached to the back of the front edge of the eighth tergite (yellow in Fig. 10), but clearly in front of the rear edge of the seventh tergite. The paired defensive glands (white in Fig. 10) are reminiscent of the lower part of rompers in their expanded form. They open close to each other through the synovium between the seventh and eighth tergite to the outside (arrowhead in Fig. 10). When alive, the glands are so shortened that they are essentially below the seventh tergite.
At the distal end of the defensive glands, tightly packed glandular tissue is attached to the side of the gland wall as strips. There are also some spots with glandular tissue in the middle area. The glandular tissue secretes a mixture of different chemical substances with a deterrent effect inwards. The substances are stored in precipitated form mainly in the middle and basal part of the glandular sac. If the beetle is strongly irritated, for example by pinching, it erects its abdomen and presses it together. This increases the pressure on the lymph, which in turn pushes the two glands outwards. In doing so, the basal parts of the gland turn inside out, comparable to a prolapsed intestine . First of all, the content stored in the basal and central area reaches the outside in the form of whitish substances. Immediately afterwards, the beetle excretes a drop of liquid from the intestine, whereby the substances are distributed over the entire tip of the abdomen. Now the beetle hits the troublemaker with the tip of its abdomen. The beetle can hit the abdomen over 90 ° upwards. The abdomen can also be tapped down, almost reaching the head. In addition, some lateral distraction of the abdomen is possible. The intestines are often emptied to strengthen the immune system. In the experiment it was shown that at least the ant species Formica exsectoides can be repelled effectively.
The everted gland can be pulled back in by muscles.
Reproduction
When two animals meet for the first time, they briefly touch each other with feelers and mouthparts, quickly rubbing them together. Obviously, this is used to determine gender. In the following, same-sex animals viciously snap against each other, possibly get involved in a short fight and then separate quickly and widely. In the case of animals of different sex, however, the male immediately tries to ride the female up. Often times the female tries to prevent this by running away quickly or by lifting her abdomen. However, the male usually only stops harassing the female after long and unsuccessful attempts.
If the female allows, the male mounts the female from behind. Then it tries to touch the slightly raised abdominal tip of the female with the slightly lowered tip of the abdomen, while at the same time it expands the genital organs. While the penis is being inserted into the female genital tract, the male's forelegs rest on the female's shoulders, with the claw member protruding over the edge of the wing cover and the rest of the tarsal limbs lying wide of the wing cover. The middle pair of legs is supported either on the edge of the abdominal segments or on the ground. The male positions the hind legs to the side of the female's abdomen on the ground. However, this hug posture is not kept for long. Occasionally during copulation, the female begins to move and the male tries to maintain his precarious position. Usually, however, the female will remain still for a few seconds. However, when it starts moving, the male slips and as a result, the male and female are positioned in almost opposite directions. As a rule, regardless of gender, the larger partner drags the weaker one behind him for some time, whereby the partners are only connected to the sexual organs. The weaker partner rarely tries to oppose the stronger one, namely to pull him away on his part.
Copulation can last over an hour. During this time, the partners often prey on and eat several fly larvae until the copula loosens. Occasionally a second male tries to mate with an already mated female. The two males then attack each other aggressively with their upper jaws. If the copula becomes detached, the stronger male tries to mate with the female. In the absence of females, males behave completely disinterested in one another.
In captivity when there is little space, the males try to copulate with the slightest contact with females, even if they have mated the same female immediately before. The copula then only lasts three to five minutes.
development
It takes about two days from egg-laying to hatching of the larvae. The larval stages up to pupation are completed in about twenty-three days in summer. The pupal stage lasts an average of twelve days. The species therefore needs an average of 37 days for development. In the laboratory, the average development times at 50% humidity and 12 hours of exposure per day at 16 ° were 1523 hours (64.4 days), at 24 ° around 857 hours and at 32 ° 571 hours (23.8 days). The larvae of the first stage are six to nine millimeters long, those of the second stage ten to fifteen millimeters. In the third and final stage, the larva becomes fifteen to 25 millimeters long and thus even slightly larger than the fully developed insect. An exact compilation of the duration of each developmental stage in the laboratory at seven different temperatures between 15 ° and 30 ° separately for egg, 1st, 2nd, 3rd larval stage and pupa, each separately for male and female, can be found in Frątczak-Łagiewska and Matuszewski .
The eggs are exceptionally laid on the ground, but usually one to five centimeters below the surface of the earth. The female does not look for a suitable place for long, but rather presses the abdomen on the ground, whereupon the tip of the abdomen penetrates deeper or not at all into the substrate, depending on the nature of the soil. This takes less than a minute and the female does not in any way check the result of the oviposition, but simply runs away. Nevertheless, this type of egg-laying makes sense, as the animals mate close to carrion and the soil there is mostly loosened by the liquid produced during the decomposition process and other animals attracted by the carrion. The larvae usually remain in the soil. Before pupation, the larva shortens and thickens, pressing an oval cavity into the ground. In it it remains in a hunched position for two to three days until pupation.
In the laboratory experiment, females fed with larvae of blowflies laid eggs more and longer than females fed with larvae of housefly . Well-nourished females laid an average of eight eggs a day, poorly fed females a little over two eggs per day. A female can lay up to around 500 eggs. When comparing three degrees of moisture, egg-laying and hatching success were independent of moisture, but the larvae preferred a substrate of four to six percent moisture over a substrate with only two percent moisture.
distribution
In Europe one finds only the subspecies C. maxillosus maxillosus . This is widespread in almost all of Europe and also in the Palearctic . The same subspecies is also reported in eastern North America and South America ( Chile , Argentina , Peru ) and most of the Atlantic islands. Most likely, these occurrences are due to human carry-over. The subspecies C. maxillosus villosus, however, shows a predominantly nearctic distribution. This subspecies is widespread in North America , the Aleutian Islands and Hawaii , to the south it radiates to Mexico , Central America , and the West Indies with Cuba , Jamaica , the Dominican Republic . If one considers Creophilus arctica as a synonym of C. maxillosus villosus , then the subspecies is also represented in the Palearctic region, namely on the Kamchatka peninsula .
literature
- Heinz Freude : The Beetles of Central Europe . Ed .: Karl Wilhelm Harde , Gustav Adolf Lohse . tape 4 Staphylinidae I. Elsevier, Spektrum, Akad. Verl., Munich 1964, ISBN 3-8274-0678-1 . P. 192
- Edmund Reitter : Fauna Germanica, the beetles of the German Empire , Volume II, KGLutz 'Verlag, Stuttgart 1909, p. 116
- Gustav Jäger (Ed.): CG Calwer's Käferbuch. K. Thienemanns, Stuttgart 1876, 3rd edition, p. 144 as Styphylinus maxillosus
Individual evidence
- ↑ a b Creophilus maxillosus and Creophilus maxillosus maxillosus from Fauna Europaea, accessed on January 6, 2019.
- ↑ a b c d Dave J. Clarke: Testing the phylogenetic utility of morphological character systems, with a revision of Creophilus Leach (Coleoptera, Staphylinidae) in ZOOLOGICAL Journal of the Linnean Society Volume 163, Number 3, November 2011, doi : 10.1111 / j.1096-3642.2011.00725.x .
- ↑ a b Carolus Linnaeus: Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis 1st volume, 10th edition, Stockholm 1758 p. 425: 421 No. 3 maxillosus .
- ↑ Carolus Linnaeus: Fauna Svecica .... Stockholm 1746 p. 192, No. 603 Staphylinus ...
- ↑ JG Wood: Insects at home . New York 1872, p. 76 maxillosus = large-jawed .
- ↑ Der Käfersammler Illustrierte Taschenbücher für die Jugend Volume 22, Ed.: Redaktion des Guten Kameraden, edited by Alexander Bau, 8th edition, Stuttgart, Berlin, Leipzig 1911, German name p. 21 .
- ↑ Jiří Zahradník: Illustrated Lexicon of Käfer Dörfler Verlag © AVENTINUM sro-Prague
- ↑ Thomas Moufet: Insectorum sive minimorum animalium theatrum , 1934, p. 197 .
- ↑ Carolus Linnaeus: Systema naturæ sistens regna tria naturæ, in classes et ordines, genera et species redacta 9th edition, Leiden (Lugduni Batavorum) 1756, p. 64, no. 179 Staphylinus .
- ^ WE Leach in G. Samouelle: The entomologist's useful compendium; or an introduction to the knowledge of British insects comprising the best means of obtaining and preserving them, and a description of the apparatus generally used; together with the genera of Linné, and the modern method of arranging the classes Crustacea, Myriapoda, spiders, mites and insects from their affinities and structure, according to the views of Dr. Leach. Also an explanation of the terms used in entomology: a calendar of the times of appearance, and usual situations of near 3000 species of British insects; with instructions for collecting and fitting up objects for the microscope London 1819 ( p. 172 Creophilus ).
- ^ Sigmund Schenkling: Explanation of the scientific beetle names from Reitterʼs Fauna Germanica . KG Lutz, Stuttgart 1917. Genera and sub-genera .
- ^ A b c John O. Westwood: An Introduction to Modern Classification of Insects . Volume 1, London 1839 ( illustration on p. 160 and object depicted in illustration questionable ).
- ↑ a b c d A. Kemner: contributions to the knowledge of some Swedish Koleopterenlarven I . In: Arkiv för Zoologi . Volume 7, number 31, Uppsala / Stockholm, p. 16 ff Creophilus maxillosus and p. 20 Evaluation of Westwood's drawing .
- ^ John Richardson: Fauna Boreali-Americana . Northern Zoology Part IV, Norwich 1837 ( p. 95 differences ).
- ^ Albert Fauvel: Synopsis of Creophilus . In: Tijdschrif voor entomologie . 18th part, 'Sgravenhaage 1875 ( p. 54f key variants ).
- ↑ HC Küster: The beetles of Europe - described from nature . 26th issue, Nuremberg 1853, not fully paged, Staphylinus maxillosus .
- ↑ Ludwig Ganglbauer : The beetles of Central Europe . Volume 2, Vienna 1895 ( Creophilus maxillosus p. 415 ).
- ↑ WF Erichson: Natural history of the insects of Germany 1st division Coleoptera . 2nd volume, Berlin 1856 ( p. 528 ).
- ↑ Richard E. Blackwelder: Morphology of the coleopterous family Staphylinidae . In: Smithsonian Miscellaneous Collections . Volume 94, Publication 3343, Washington 1936 ( biodiversitylibrary.org )
- ↑ Pictures of larvae: bugguide.net biodiversidadvirtual.org , biodiversidadvirtual.org .
- ↑ a b c d e f g Cyril E. Abbott: The development and general biology of Creophilus villosus Gray . In: Journal of the New York Entomological Society . Volume 46, New York 1938, pp. 49-53, JSTOR 25004733 ( pp. 49 ff ).
- ↑ picture of a doll .
- ↑ Sergey Victorovich Pushkin: Necrobionts an Necrophilous Beetles (Insecta; Coleoptera) of the South of the Russia . In: World Applied Sciences Journal . Volume 32, number 4, 2004, pp. 618-625, doi : 10.5829 / idosi.wasj.2014.32.04.14517 , pp. 6/623
- ↑ Erin J. Watson-Horzelski, Anna C. Clark-Aguilard: Predatory Behavior of Creophilus maxillosus (L.) (Coleoptera: Staphylinidae) towards the invasive Blow Fly Chrysomaya rufifacies (Macquart) (Diptera: Calliphoridae) . In: The Coleopterists Bulletin . Volume 65, number 2, pp. 177-182, doi : 10.1649 / 072.065.0218 .
- ↑ George S. Fichter: Necrophily vs. Necrophagy . In: Ohio Journal of Science . Volume 49, Number 5, September 1949, pp. 201-204, hdl : 1811/3719 .
- ^ Szymon Matuszewski: Estimating the Preappearance Interval from Temperature in Creophilus maxillosus L. (Coleoptera, Staphylinidae) . In: Journal of Forensic Sciences . Volume 57, number 1, January 2012, pp. 136-145, doi : 10.1111 / j.1556-4029.2011.01958.x .
- ↑ a b Erin J. Watson-Horzelski: Survival and time of development for Creophilus maxillosus (L.) (Coleoptera, Staphylinidae) at three constant temperatures . In: Coleopterists Bulletin . Volume 66, Number 4, December 2012, pp. 365-370, JSTOR 41819746 .
- ↑ Sz. Matuszewski, D. Bajerlein, Sz. Konwerski, K. Szpila: Insect succession and carrion decomposition in selected forests in Central Europe, Part 3: Succession of carrion fauna . In: Forensic Science International . Volume 207, April 15, 2011, pp. 150-163, doi : 10.1016 / j.forsciint.2010.09.022 .
- ↑ S. Vanin, E. Zanotti, D. Gibelli, A. Taborelli, S. Andreola, C. Cattaneo: Dekomposition and entomological colonization of charred bodies - a pilot study . In: Croatian Medical Journal . Volume 54, Number 4, August 2013, PMC 3760664 (free full text).
- ↑ a b Szymon Matuszewski, Michał Szafałowicz: Temperature-dependent appearence of forensically useful beetles on carcasses . In: Forensic Science International . Volume 229, number 1–3, June 10, 2013, pp. 92–99, doi : 10.1016 / j.forsciint.2013.03.034 ( PDF ).
- ↑ Sz. Matuszewski, K. Frątczak-Łagiewska: Size at emergence improves accuracy of age estimates in forensically-useful beetle Creophilus maxillosus L. (Staphylinidae) . In: Scientific Reports , Volume 8, 2018, Article 2390, doi : 10.1038 / s41598-018-20796-1 .
- ↑ Thomas Eisner, Maria Eisner, Melody Siegler: Secret Weapons . Cambridge, Massachusetts / London, England 2005 ( preview in Google Book search)
- ↑ M. Jefson, J. Meinwald, S. Nowicki, K. Hicks, Th. Eisner: Chemical defense of a rove beetle (Creophilus maxillosus) . In: Journal of Chemical Ecology . Volume 9, number 1, pp. 159-180, doi : 10.1007 / BF00987779 .
- ↑ Angela Huth, Konrad Dettner: Chemical defense of the great fledglings from the subtribe Staphylinina (Col .: Staphylinidae) . In: Communications of the German Society for General and Applied Entomology e. V. Giessen 1989, ( PDF )
- ↑ Fr. Dierckx: Les glandes pygidiennes des Staphylinides et Cicindelides . In: Zoologischer Anzeiger . Volume 22, Leipzig 1899, p. 311 ff
- ^ A b George S. Fichter: Notes on the mating behavior and oviposition of Creophilus maxillosus (Linné) . In: Entomological News . Volume 60, pp. 175-178, Philadelphia Pennsylvania, 1938 ( pp. 175 ff ).
- ↑ K. Frątczak, Sz. Matuszewski: Instar determination in forensically useful beetles Necrodes littoralis (Silphidae) and Creophilus maxillosus (Staphylinidae) . In: Forensic Science International . Volume 241, 2014, pp. 20-26, doi : 10.1016 / j.forsciint.2014.04.026 ( abstract ).
- ↑ Katarzyna Frątczak-Łagiewska, Szymon Matuszewski: Sex-specific developmental models for Creophilus maxillosus (L.) (Coleoptera: Staphylinidae): searching for larger accuracy of insect age estimates . In: International Journal of Legal Medicine . Volume 122, number, 3, May 2018, pp. 887-885, doi : 10.1007 / s00414-017-1713-4 .
- ↑ Gerald L. Greene: Rearing Techniques for Creophilus maxillosus (Coleoptera, Staphylinidae) a Predator of Fly Larvae in Cattle Feedlots in Oxford Acadamic, Journal of Entomology Vol 89 Issue 4 August 1996, doi : 10.1093 / jee / 89.4.848 .