Danishefsky-Dien
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Danishefsky-Dien | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 16 O 2 Si | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 172.30 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.89 g cm −3 (20 ° C) |
|||||||||||||||
| boiling point |
68-69 ° C (18.9 hPa) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The Danishefsky diene (after Samuel Danishefsky ) is a chemical compound from the class of dienes . It has the basic structure of 1,3-butadiene , which has a methoxy group in the 1-position and a trimethylsilyl ether in the 3-position .
properties
It is a colorless liquid with a refractive index of 1.454 (20 ° C). The flash point is 59 ° C.
Manufacturing
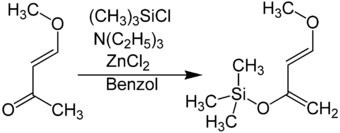
The Danishefsky diene can be synthesized from commercially available trans -4-methoxybut-3-en-2-one . This is initially by a base , for example triethylamine , deprotonated . The resulting enolate is stabilized with zinc chloride and converted to Danishefsky diene with chlorotrimethylsilane .
use
The Danishefsky diene is used for the Diels-Alder reaction . It enables the synthesis of products with a carbonyl function in a position that are not accessible in a Diels-Alder reaction with other dienes. This is controlled by the work-up: After the Diels-Alder reaction, the silyl ether can be split off with dilute hydrochloric acid , which initially produces an enol , which then tautomerizes to the ketone . The resulting ketone can be a useful functionalization for subsequent reaction steps. The methoxy group can also be split off under acidic conditions if it is not desired, with a double bond resulting.

The Danishefsky diene is an electron- rich diene and shows a high regioselectivity in reactions . The most electron-rich position is at C-1, to which the methoxy group is attached. If asymmetrical dienophiles are used, this position reacts preferentially with the electron-poor position of the dienophile used. The Danishefsky diene can also be used in hetero Diels-Alder reactions . An example of this is an aza-Diels-Alder reaction, in which the C = N double bond of an imine reacts as a dienophile:

variants
There are also variations of the Danishefsky service. In most cases, other residues are used on the silyl ether and on the ether. These are summarized under the collective term Danishefsky-Diene.
Web links
Individual evidence
- ↑ a b c Datasheet Danishefsky-Dien (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b c data sheet trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene from Sigma-Aldrich , accessed on May 25, 2011 ( PDF ).
- ↑ S. Danishefsky, T. Kitahara, J. Am. Chem. Soc. 1974 , 96 , 7807-7808.