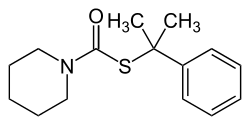
Dimepiperate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimepiperate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 15 H 21 NOS | |||||||||||||||
| Brief description |
waxy solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 263.40 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.08 g cm −3 (25 ° C ) |
|||||||||||||||
| Melting point |
38.8-39.3 ° C |
|||||||||||||||
| boiling point |
164-168 ° C / 0.75 mmHg |
|||||||||||||||
| Vapor pressure |
0.53 mPa (30 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water: 20 mg l −1 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dimepiperate is a chemical compound from the group of thiocarbamates , which was developed by Mitsubishi Petrochemical (today Nihon Nōyaku ) and marketed as a herbicide by Rhône-Poulenc Yuka Agro .
Extraction and presentation
Dimepiperate can be obtained starting from piperidine by reacting with phosgene and dimethylbenzyl mercaptan .
use
Dimepiperat is used as a herbicide in rice cultivation to control the chicken millet and also as a herbicide safener together with bensulfuron-methyl (trade name Push ).
Admission
No plant protection products containing this active ingredient are permitted in the EU or Switzerland .
Individual evidence
- ↑ a b c d e f g data sheet Dimepiperate at 9ele.
- ↑ a b c d e f Entry on dimepiperate in the GESTIS substance database of the IFA , accessed on May 17, 2014 (JavaScript required)
- ↑ Entry on S- (1-methyl-1-phenylethyl) piperidine-1-carbothioate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 105 ( limited preview in Google Book search).
- ↑ Joanna Davies, John C. Caseley: Herbicide safeners: a review . In: Pesticide Science . tape 55 , no. 11 , November 1999, p. 1043-1058 , doi : 10.1002 / (SICI) 1096-9063 (199911) 55:11 <1043 :: AID-PS60> 3.0.CO; 2-L ( PDF ).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Dimepiperate in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.


