Dimethyl succinyl succinate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimethyl succinyl succinate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 12 O 6 | |||||||||||||||
| Brief description |
white to slightly yellow-green crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 228.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
330 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1,600 (25 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Dimethylsuccinylsuccinate is a key raw material for quinacridones , a group of high-performance pigments that are characterized by their particularly high resistance to the effects of light, temperature, weather and solvents.
Occurrence and representation
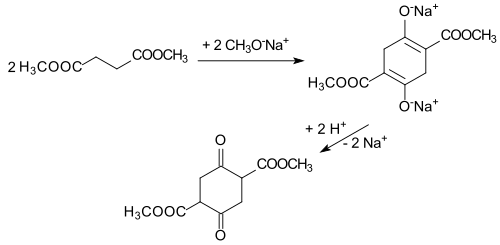
The base-catalyzed reaction of dimethyl succinate (dimethyl succinate) to dimethyl succinylsuccinate - referred to here as dimethyl succinic succinic acid - was first described in 1885, which was later referred to as Claisen condensation .
In a process that is archaic by today's standards using granulated sodium in methyl succinate and a few drops of methanol, pure DMSS was obtained in less than 50% yield after a four-week waiting period. In the following 100 years it was possible to reduce product losses due to side reactions and during work-up and the yield increased to approx. 75%.
A more recent variant describes a synthesis route with isolation of the intermediate stage of the disodium salt and delivers pure yields of approx. 85% even in industrial approaches.
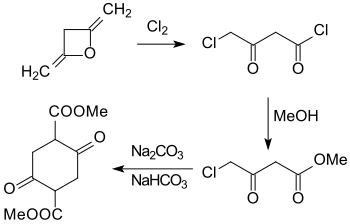
An alternative route via the cyclization of methyl 4-haloacetoacetate - from diketene and chlorine with subsequent reaction of the 4-chloroacetoacetyl chloride obtained with methanol - to give dimethyl succinylsuccinate has not caught on.
properties
Dimethyl succinyl succinate is a white crystalline powder that is only very slightly soluble in water and dissolves in methanol and DMSO. Temperature-dependent solubilities of DMSS in various dipolar solvents are described.
Applications
Manufacture of synthesis building blocks
1,4-Cyclohexanedione is analogous to the Organic Syntheses regulation and can also be obtained with DMSS instead of the starting material diethyl succinylsuccinate DESS.
2,5-Dihydroxyterephthalic acid can be obtained from DMSS by oxidation and ester hydrolysis
Substituted 2,5-dihydroxyterephthalic acids from DMSS are building blocks for rod - shaped liquid - crystalline polyesters with long side chains, the so-called "hairy rod polymers", which form very stable Langmuir-Blodgett layers . Polycondensation of 2,5-dihydroxyterephthalic acid with 2,3,5,6-tetraaminopyridine produces polymers from which fibers with very high tensile strengths can be spun.
Synthesis of quinacridone and quinacridonequinone pigments
By far the most important application of dimethyl succinylsuccinate is as a building block for the pigment group of the quinacridones and quinacridonequinones.
In 1935 Hans Liebermann - who lost his job at the TH Berlin as a Jew in 1938 and then committed suicide - published the synthesis of linear quinacridones, starting from 2,5-dianilinoterephthalic acid based on dimethylsuccinylsuccinate. The colors of the "quinacridones" obtained at that time from yellow to red to violet were specified, but their extraordinary product properties as pigments were not recognized.
In the first stage of the reaction to quinacridone, DMSS is reacted with aniline under nitrogen and acid catalysis to give dimethyl 2,5-dianilino-3,6-dihydroterephthalate in practically quantitative yield. The dihydroterephthalic acid diester can then be oxidized to the terephthalic acid diester with atmospheric oxygen. In a one-pot reaction, the condensation with aniline in acid and the subsequent oxidation with air in alkaline in the presence of cationic surfactants takes place practically quantitatively to 2,5-dianilinoterephthalic acid. Dianilinoterephthalic acid is obtained by heating in the presence of dehydrating agents, such as. B. polyphosphoric acid converted into quinacridone.
The by oxidation of the central benzene ring in the quinacridone, z. Example by means of potassium , resulting golden yellow to maroon (engl. Maroon ) quinacridonequinones
can also be obtained by chlorination of dimethylsuccinylsuccinate, reaction of the 3,6-dichloro-1,4-benzoquinone derivative with anilines and thermal cyclization
literature
- K. Hunger, MU Schmidt, T. Heber: Industrial Organic Pigments: Production, Crystal Structures, Properties, Applications, 4th Edition . Wiley-VCH, Weinheim 2018, ISBN 978-3-527-32608-2 .
- EB Faulkner, RJ Schwartz (Ed.): High Performance Pigments, 2nd Edition . Wiley-VCH, Weinheim 2009, ISBN 978-3-527-31405-8 .
Individual evidence
- ↑ Entry on Dimethyl-1,4-cyclohexanedione-2,5-dicarboxylate at TCI Europe, accessed on April 8, 2019.
- ^ A b c d Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Amsterdam, NL 2016, ISBN 978-0-323-28659-6 , pp. 286 .
- ↑ Data sheet dimethyl-2,5-dioxocyclohexane-1,4-dicarboxylate from Sigma-Aldrich , accessed on April 8, 2019 ( PDF ).
- ↑ a b c d e data sheet Dimethyl-1,4-cyclohexanedione-2,5-dicarboxylate from AlfaAesar, accessed on April 8, 2019 ( PDF )(JavaScript required) .
- ↑ H. Ebert: On the Constitution of Succinylobernsteinsäureäthers . In: Liebigs Ann. Chem. Band 229 , no. 1–2 , 1885, pp. 45-88 , doi : 10.1002 / jlac.18852290103 .
- ↑ Patent EP0057873A1 : Process for the production of dimethyl succinylosuccinate, its disodium salt, dianilinodihydroterephthalic acids, their dimethyl esters and salts and of dianilinoterephthalic acids, their dimethyl esters and salts. Applied on August 18, 1982 , published on January 29, 1982 , applicant: Bayer AG, inventor: M. Rolf, D.-I. Schütze, R. Neeff, H.-V. Runzheimer's.
- ↑ Patent US5783723 : Process for the preparation of dialkyl succinylsuccinates. Filed September 24, 1996 , published July 21, 1998 , Applicant: Ciba Specialty Chemicals Corp., Inventor: CD Campbell, DT Cole, HR Taylor.
- ↑ Patent DE2313329 : Process for the production of succinyosuccinic diester . Registered on March 17, 1973 , published October 4, 1973 , applicant: Lonza AG, inventor: E. Greth.
- ↑ Patent EP2518043A1 : Process for the production of 4-chloroacetyl chloride, 4-chloroacetic acid esters, amides and imides. Applied on April 29, 2011 , published on October 31, 2012 , applicant: Lonza Ltd., inventor: NN.
- ↑ Z.-G. Chen, W.-G. Yang, Y.-H. Hu, Z.-Y. Lei, Y.-H. Wang, W.-Q. Wan: Measurement and Correlation for the Solubility of dimethyl 1,4-Cyclohexanedione-2,5-dicarboxylates in Different Solvents at Temperatures from (278.15 to 323.15) K . In: J. Chem. Eng. Data . tape 56 , no. 6 , 2011, p. 2726-2729 , doi : 10.1021 / je2000292 .
- ^ AT Nielsen, WR Carpenter: 1,4-Cyclohexanedione In: Organic Syntheses . 45, 1965, p. 25, doi : 10.15227 / orgsyn.045.0025 ; Coll. Vol. 5, 1973, p. 288 ( PDF ).
- ↑ a b J. Li, Z. Hu, Y. Song, Y. Huang, VV Zuev: New route to poly (2,6-diimidazo (4,5-b: 4 ', 5'-e) pyridinelene-1 , 4-dihydroxy-phenylene) (PIPD) and high modulus fiber on its basis . In: Nanosystems: Phys. Chem. Math. Band 5 , no. 6 , 2014, p. 829-835 ( ifmo.ru [PDF]).
- ↑ M. Ballauff: Rigid rod polymers having flexible side chains, 1. Thermotropic poly (1,4-phenylene 2,5-dialkoxyterephthalate) s . In: Macromol. Rapid Commun. tape 7 , no. 6 , 1986, pp. 407-414 , doi : 10.1002 / marc.1986.030070615 .
- ↑ H. Liebermann: About the formation of quinacridones from p-di-arylamino-terephthalic acids. 6. Communication on conversion products of the succinylosuccinic acid ester . In: Liebigs Ann. Chem. Band 518 , no. 1 , 1935, p. 245-259 , doi : 10.1002 / jlac.19355180115 .
- ↑ Patent EP0536083A1 : Process for the synthesis of dialkyl succinylsuccinate esters and their conversion to dialkyl-2,5-diarylamino-3,6-dihydroterephthalate esters. Applied on September 22, 1992 , published on April 7, 1993 , applicant: Ciba-Geigy AG, inventor: CD Campbell, EE Jaffe.
- ↑ Patent DE3834747A1 : Process for the production of 2,5-diarylaminoterephthalic acids. Registered on October 12, 1988 , published on May 3, 1990 , applicant: Bayer AG, inventor: D.-I. Schütze, R. Schmitz.
- ↑ a b Patent US6972333B2 : Preparation of quiacridonequinones and substituted derivatives of the same. Filed January 14, 2004 , published December 6, 2005 , applicant: Sun Chemical Corp., inventor: EH Sung, JZ Dong, GH Robertson.
- ^ W. Herbst, K. Hunger: Industrial Organic Pigments: Production, Properties, Applications, 3rd Edition . Wiley-VCH, Weinheim 2004, ISBN 3-527-30576-9 , pp. 459 .