Diphenoxylate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
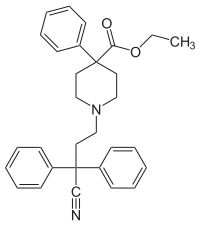
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Diphenoxylate | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 30 H 32 N 2 O 2 | ||||||||||||||||||
| Brief description |
white crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Opioid , motility inhibitor |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 452.59 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
221–222 ° C (as hydrochloride ) |
||||||||||||||||||
| pK s value |
7.1 |
||||||||||||||||||
| solubility |
Water: 0.8 g l −1 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Diphenoxylate is an opiate agonist with peristalsis-inhibiting effect and is used as a medicinal substance for the treatment of diarrhea .
clinic
Diphenoxylate and its main metabolite, difenoxine, contain structural elements of the methadone and pethidine series. As opioids - especially in high doses or in long-term treatment - they are potentially addictive. For this reason, a pharmacological strategy was developed and the drug was combined with atropine sulfate. The anticholinergic effects of atropine lead to weakness and nausea when the standard dose is exceeded. Despite the structural similarity, this addition is not necessary with loperamide because it does not reach the central nervous system (CNS).
pharmacology
The anti-peristalsis effect of diphenoxylate and its metabolite difenoxin is based on their reaction with the opiate receptors of the intestine. There is also a hydroxylation product , some of which is excreted as a conjugate . Diphenoxylate is rapidly absorbed after ingestion, the plasma half-life is 2.5 hours, that of the free acid (difenoxine) is 4.4 hours. It is excreted preferentially in the faeces and about 10% renally .
Law
Diphenoxylate was patented in 1959 as an antidiarrheal by the Belgian pharmaceutical company Janssen Pharmaceutica . Diphenoxylate is listed as a marketable but not prescribable substance in BtMG 1981 Annex II (to Section 1, Paragraph 1). A prescription is possible as long as the preparation does not contain more than 0.25% or the derived form does not contain more than 2.5 mg of diphenoxylate and (based on this amount) at least 1% atropine sulfate is added.
- Switzerland : With the entry into force of the revised Narcotics Ordinance by Swissmedic on December 1, 2010, diphenoxylate will be subject to the Narcotics Act.
Trade names
Tropergen (GB), Lomotil (USA), Diarsed (F), Reasec (Cz)
literature
- Auterhoff , Knabe, Höltje: Textbook of Pharmaceutical Chemistry . 13th edition. Knowledge. Verl. Ges., Stuttgart 1994, ISBN 3-8047-1356-4 .
Individual evidence
- ↑ a b Entry on diphenoxylate. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ Sean Sweetman (Editor): Martindale: The Complete Drug Reference, 35th Edition: Book and CD-ROM Package . Pharmaceutical Press, ISBN 978-0-85369-704-6
- ↑ Entry on diphenoxylate in the DrugBank of the University of Alberta .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Entry on diphenoxylate in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Text of the Swissmedic Narcotics Ordinance, which came into force on December 1, 2010, as a PDF .
- ↑ Text of the Swiss Narcotics Act as PDF .