Phosphorus pentoxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phosphorus pentoxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | P 4 O 10 | |||||||||||||||
| Brief description |
colorless, odorless, hygroscopic solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 283.92 g mol −1 (P 4 O 10 ) | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| Sublimation point |
362 ° C (under normal pressure) |
|||||||||||||||
| solubility |
decomposes to phosphoric acid with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phosphorus pentoxide , more precisely diphosphorus pentoxide , is an oxide of the element phosphorus and belongs to the group of phosphorus oxides . It is a colorless, odorless, extremely hygroscopic powder that is converted to phosphoric acid with water in a strongly exothermic reaction .
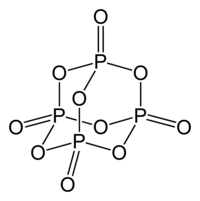
Although it has long been known that the molecule can be described by the empirical formula P 4 O 10 , the historical name (di) phosphorus pentoxide , i.e. P 2 O 5 , has been retained.
Manufacturing
Phosphorus pentoxide is formed when white phosphorus is burned in a dry stream of air with strong heat development:
In the case of a lack of oxygen, phosphorus trioxide P 4 O 6 is also produced , in which the four “outer” oxygen atoms (in the above formula, double bonded to a phosphorus atom) are missing.
properties
Physical Properties
Phosphorus pentoxide sublimes at 362 ° C , the melting point can only be determined under pressure in a closed ampoule as 562 ° C.
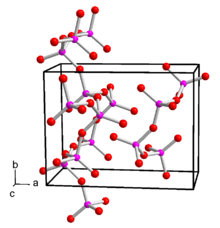
Phosphorus pentoxide is polymorphic . The conversion of a hexagonal H-shape takes place at 450 ° C under pressure over 24 hours via the liquid and glass phase to the orthorhombic O-shape and further to the stable, likewise orthorhombic O'-shape. The orthorhombic form, which is stable at room temperature, crystallizes with the space group space group Pnma (space group no. 62) and the lattice parameters a = 9.193 Å, b = 4.89 Å and c = 7.162 Å. This consists of infinite layers made up of six rings of PO 4 tetrahedra. There is also a metastable trigonal structure with the space group R 3 c (No. 161) and the lattice parameters a = 10.2 Å and c = 13.5 Å. This consists of isolated P 4 O 10 molecules. There is also the o-form, which does not consist of isolated molecules, but of chains of PO 4 tetrahedra linked by three oxygen atoms . This shape is also orthorhombic, space group Fdd 2 (No. 43) , with the lattice parameters a = 16.31 Å, b = 8.115 Å and c = 5.265 Å. In the gas phase there are P 4 O 10 molecules. By means of electron diffraction , the bond lengths of the P = O double bond could be determined as 143 pm and the PO single bond as 160 pm. The bond angles are 123.5 ° for POP, 116.5 ° for O = PO and 101.6 ° for OPO.
Chemical properties
Phosphorus pentoxide has a great tendency to absorb water and form tetrametaphosphoric acid :
The equilibrium is almost completely on the right-hand side, so that a water vapor pressure of less than 1.3 · 10 −4 Pa is established in a closed space containing phosphorus pentoxide .
Further water uptake is via the simplest polyphosphoric acid, diphosphoric acid
The gross response
is strongly exothermic. Here a molar heat of reaction of −377 kJ mol −1 is realized. Phosphorus pentoxide also splits water from compounds. Thus resulting from nitric acid , the anhydride of dinitrogen pentoxide , from perchloric acid , the dichlorine heptoxide , from sulfuric acid , the sulfur trioxide and from malonic acid , the carbon suboxide .
use
Due to the equilibrium presented above, phosphorus pentoxide is an extremely effective drying agent (see desiccator ): water molecules from the surrounding air that come into contact with phosphorus pentoxide are very strongly bound. Commercial desiccants contain 75% phosphorus pentoxide and 25% inert inorganic carrier material, which means that the desiccant remains free-flowing. The degree of exhaustion of the agent is indicated by adding water indicators. When water is absorbed, the originally colorless indicator changes color from green to blue-green to blue (water content approx. 33%).
Water contained in gases, liquids and solids has a higher water vapor pressure, as a result of which the water molecules gradually migrate almost completely to the phosphorus pentoxide. This also affects chemically bound water (see crystal water ) and even separately bound hydrogen and hydroxyl groups in organic molecules or biological material: These also have a certain water vapor pressure, i.e. an OH group occasionally forms a water molecule with an adjacent hydrogen atom that evaporates when the air is extremely dry. This equilibrium does not play a role in the air that surrounds us and contains water vapor, but a lump of sugar in the desiccator turns black like the cake forgotten in the oven (which lost the water in the same way due to the heat).
Fertilizers contain phosphates such as B. potassium dihydrogen phosphate KH 2 PO 4 or diammonium hydrogen phosphate (NH 4 ) 2 HPO 4 , the content of phosphorus is often given in terms of phosphorus pentoxide (P 2 O 5 ).
Although phosphorus pentoxide is practically only used as a drying agent in organic chemistry , it has been shown experimentally that the cation of phosphorus pentoxide is so reactive in the gas phase that it is even the most stable of all carbon-hydrogen bonds, namely that of methane , effectively can activate at room temperature .
The compound is used industrially for the production of very pure thermal phosphoric acid and for the synthesis of organic phosphoric acid esters . The implementation with ethers leads to the Trieste.
Mono- and diesters are formed with alcohols .
safety instructions
Phosphorus pentoxide causes severe burns . Therefore gloves, respiratory protection and appropriate laboratory clothing must be worn when handling.
Individual evidence
- ↑ a b c d e f g h i Entry on phosphorus pentoxide in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e f g h i Wiberg, E .; Wiberg, N .; Holleman, AF : Inorganische Chemie , 103rd edition, 2017 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-026932-1 , pp. 898-899, (accessed via De Gruyter Online).
- ↑ Entry on Diphosphorus pentaoxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 1314-56-3 or phosphorus pentoxide ), accessed on November 2, 2015.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 644.
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. 2nd Edition. vol. 1, Academic Press, 1963, pp. 541-542.
- ↑ D. Stachel, I. Svoboda, H. Fuess: Phosphorus Pentoxide at 233 K. In: Acta Crystallographica , C51, 1995, pp. 1049-1050, doi: 10.1107 / S0108270194012126 .
- ^ DWJ Cruickshank: Refinements of structures containing bonds between Si, P, S or Cl and O or NV P 4 O 10 . In: Acta Crystallographica , 17, 1964, pp. 677-679, doi: 10.1107 / S0365110X64001670 .
- ↑ EH Arbib, B. Elouadi, JP Chaminade, J. Darriet: New refinement of the crystal structure of oP 2 O 5 . In: Journal of Solid State Chemistry , 127, 1996, pp. 350-353, doi: 10.1006 / jssc.1996.0393 .
- ↑ a b c d e f g Ralf Steudel : Chemistry of Non-Metals, Syntheses - Structures - Binding - Use , 4th edition, 2014 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-030439- 8 , pp. 408-409, (accessed via De Gruyter Online).
- ↑ K. Schrödter, G. Bettermann, T. Staffel, F. Wahl, T. Klein, T. Hofmann: Phosphoric Acid and Phosphates. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim 2008. doi : 10.1002 / 14356007.a19_465.pub3
- ↑ R. Crabtree: C-H bond activation: A radical non-metal solution. In: Nature Chemistry . 1, 2009, pp. 348-349. (Original work: N. Dietl, M. Engeser. H. Schwarz: Activation of the C – H bond of methane at room temperature by naked [P 4 O 10 ] + . In: Angewandte Chemie. 121, 2009, pp. 4955-4957 doi: 10.1002 / anie.200901596 )