Phosphorus trioxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phosphorus trioxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | P 4 O 6 | |||||||||||||||
| Brief description |
Waxy, colorless, monoclinic crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 109.96 g mol −1 or 219.92 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.13 g cm −3 |
|||||||||||||||
| Melting point |
23.8 ° C |
|||||||||||||||
| boiling point |
175.3 ° C |
|||||||||||||||
| solubility |
good at benzene and carbon disulfide |
|||||||||||||||
| Refractive index |
1.540 (27 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Phosphorus trioxide is an oxide of the element phosphorus . This chemical compound is also known as diphosphorus trioxide . The molecular mass determined in the molten, dissolved and vaporous state corresponds to the empirical formula P 4 O 6 . The waxy, colorless crystals are very poisonous .
presentation
Phosphorus trioxide is produced when white phosphorus is burned at low temperatures with a lack of oxygen . A strong development of heat is observed:
- White phosphorus reacts with oxygen to form phosphorus trioxide.
properties
Physical Properties
Phosphorus trioxide is the anhydride of phosphonic acid . Phosphorus is in the +3 oxidation state. The crystals of this compound have a density of 2.14 g · cm −3 . The melting point is 24 ° C, the boiling point at 175 ° C under a nitrogen - atmosphere . Phosphorus trioxide dissolves in benzene and carbon disulfide .
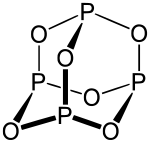
The structure of phosphorus trioxide is derived from the tetrahedral phosphorus molecule P 4 . By replacing the six P -P- bonds - the edges of the tetrahedron - respectively by P O -P bonds, we come to the illustrated above molecular structure. The bond lengths of the PO bonds are 164 pm for the molecule in the gas state. The bond angles are 126.4 ° for the POP group and 99.8 ° for the OPO group.
The structure has a high degree of symmetry . In addition to the tetrahedral arrangement of the phosphorus atoms , an octahedral arrangement can be determined for the oxygen atoms . The four faces of the tetrahedral basic structure are framed by four symmetrically linked P 3 O 3 six-membered rings. This structure is also found in arsenic (As 4 O 6 ) and urotropine (N 4 (CH 2 ) 6 ).
Phosphorus trioxide crystallizes in a monoclinic crystal structure in the space group P 2 1 / m (space group no. 11) and the lattice parameters a = 6.43, b = 7.887, c = 6.183 Å and β = 106.01 °.
Chemical properties
Phosphorus trioxide oxidized in air further phosphorus pentoxide :
- Phosphorus trioxide oxidizes to phosphorus pentoxide.
Chemiluminescence is observed during this process under reduced pressure .
Above 210 ° C a decomposition to phosphorus and phosphorus tetroxide takes place:
- Phosphorus trioxide disproportionates to phosphorus and phosphorus tetroxide.
In cold water , phosphorus trioxide is converted to phosphonic acid :
- Phosphorus trioxide reacts with water to form phosphonic acid.
The disproportionation of phosphonic acid becomes relevant in hot water, so that phosphoric acid , phosphine and phosphorus are then obtained as reaction products.
The reaction with hydrogen chloride leads to the formation of phosphonic acid and phosphorus trichloride :
- Phosphorus trioxide and hydrogen chloride react to form phosphonic acid and phosphorus trichloride.
Phosphorus trioxide reacts with the halogens chlorine and bromine to form phosphoryl halides , and with iodine to form diphosphorus tetraiodide . The reaction with ozone leads to the ozonide P 4 O 18 , which slowly decomposes above −35 ° C to form phosphorus pentoxide, releasing oxygen .
swell
- ↑ a b Entry on phosphorus oxides. In: Römpp Online . Georg Thieme Verlag, accessed April 30, 2014.
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 786.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Liquids, pp. 4-140.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d e Ralf Steudel : Chemistry of Non-Metals, Syntheses - Structures - Bonding - Use , 4th edition, 2014 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-030439-8 , Pp. 407-408, (accessed via De Gruyter Online).
- ↑ M. Jansen, M. Voss, HJ Deiseroth: Structural properties of phosphorus oxides in the solid state . In: Angewandte Chemie , 1981 , 93 , pp. 1023-1024, doi : 10.1002 / anie.19810931127 .





