EDA-DOPO
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
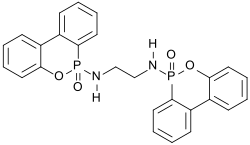
|
|||||||||||||
| General | |||||||||||||
| Surname | EDA-DOPO | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 26 H 22 N 2 O 4 P | ||||||||||||
| Brief description |
White dust |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 488.4 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
271 ° C |
||||||||||||
| solubility |
practically insoluble in water, poorly soluble in chloroform |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
EDA DOPO is a reaction product of DOPO with ethylenediamine a novel phosphonamidate, the halogen-free as a highly effective flame retardants , especially for polyurethane - foam is placed on the market.
Manufacturing
The phosphinic ester DOPO must first be activated by converting it into the acid chloride , whereby various chlorinating agents can be used. The Atherton-Todd reaction uses the carcinogenic carbon tetrachloride to chlorinate DOPO to DOP-Cl (Route A.); however, the method is obsolete because of the use of CCl 4 .
With sulfuryl chloride SO 2 Cl 2 in dichloromethane in the presence of triethylamine as the base, yields of up to 85% are achieved (preferred route B.), while a poorer yield of 74% is achieved with the solid trichloroisocyanuric acid TCCA as the chlorination reagent in acetonitrile as the solvent (route C. .). The product is obtained as a 1: 1 mixture of diastereomers .
properties
EDA-DOPO is a solid that is obtained as a white crystal powder and is hardly soluble in water and only slightly soluble in chloroform . The connection is thermally extremely stable and only begins to decompose at temperatures above 350 ° C, which is attributed to the spatial shielding of the unstable PN bond by the biphenyl structures. In-vitro tests have so far not provided any indications of neurotoxic , cytotoxic or inflammatory properties.
Applications
The DOPO precursor already has useful fire-retardant properties, but a decomposition temperature that is too low (<200 ° C), especially for thermoplastically processed polymers. The flame-retardant effect of DOPO and its derivatives is attributed to the formation of reactive radicals of the PO • and HPO • type in the gas phase formed during the pyrolysis of the material (gas phase mechanism). At EDA-DOPO, synergies between nitrogen and phosphorus for the flame-retardant effect are also discussed.
Due to its high melting and decomposition temperature, EDA-DOPO is suitable as a halogen-free flame retardant that is effective when added in relatively low amounts (<5%) for high-melting thermoplastic and thermosetting plastics such as polyester or polyamides , whereby it is classified according to the generally recognized UL 94 flammability test V0 has been classified as least flammable. EDA-DOPO can prove its effectiveness and its ecological and economic profile with the recently increased use for flame retardant finishing of polyurethane foams .
For the optimal stabilization of plastics, mixtures of flame retardants with different mechanisms of action are usually used, such as B. aluminum hydroxide , red phosphorus or organic compounds such as radical scavengers, antioxidants or intumescent materials such as ammonium polyphosphates .
literature
- Edward D. Weil, Sergei V. Levchik: Flame retardants for Plastics and Textiles - Practical Applications, 2nd edit. Carl Hanser, Munich 2015, ISBN 978-1-56990-578-4 .
- Rudolf Pfaendner, Manfred Döring: Eco-Friendly Fire Prevention . In: Kunststoffe International . Carl Hanser, Munich 2014 ( fraunhofer.de [PDF]).
Individual evidence
- ↑ a b DOPO-EDA, Technical Data Sheet. (PDF; 44 KB) In: metadynea.at. Metadynea Austria GmbH, accessed on June 27, 2018 (English).
- ↑ a b Patent EP2557085A1 : Novel phosphonamidates - synthesis and flame retardant applications. Registered on August 8, 2011 , published on February 13, 2013 , applicant: EMPA Eidgenössische Materialprüfungs- und Forschungsanstalt, F. Nauer AG, inventors: H. Mispreuve, R. Näscher, S. Gaan, M. Neisius, P. Mercoli, S. Liang.
- ↑ There is not yet a harmonized classification for this substance . A labeling of EDA-DOPO in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 1, 2018, is reproduced from a self-classification by the distributor .
- ↑ a b c N.M. Neisius, M. Lutz, D. Rentsch, P. Hemberger, S. Gaan: Synthesis of DOPO-based phosphonamidates and their thermal properties . In: Ind. Eng. Chem. Res. Volume 53 , no. 8 , 2014, p. 2889-2896 , doi : 10.1021 / ie403677k .
- ↑ C. Hirsch et al .: Multiparameter toxicity assessment of novel DOPO-derived organophosphorus flame retardants . In: Arch. Toxicol. tape 91 , no. 1 , 2017, p. 407-425 , doi : 10.1007 / s00204-016-1680-4k .
- ↑ M. Rakotomalala, S. Wagner, M. Döring: Recent developments in halogen free flame retardants for electrical and electronic applications . In: Materials . tape 3 , 2010, p. 4300-4327 , doi : 10.3390 / ma3084300 .
- ↑ Technical information, plastics, flame retardancy of plastics. In: www.bobla.de. Bobla Housing Systems GmbH, archived from the original on July 9, 2017 ; accessed on June 28, 2018 .
- ↑ A. Buczko, T. Stelzig, L. Bommer, D. Rentsch, M. Heneczkowski, S. Gaan: Bridged DOPO derivatives as flame retardants for PA6 . In: Polym. Degradation. Rod. tape 107 , 2014, p. 158–165 , doi : 10.1016 / j.polymdegradstab.2014.05.017 .
- ^ S. Liang, M. Neisius, H. Mispreuve, R. Näscher, S. Gaan: Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds . In: Polym. Degradation. Rod. tape 77 , 2012, p. 2428-2440 , doi : 10.1016 / j.polymdegradstab.2012.07.019 .
- ↑ New flame retardant before market entry. In: www.empa.ch. empa, Eidgenössische Materialprüfungs- und Forschungsanstalt, September 26, 2017, accessed on June 28, 2018 .
