Ecteinascidin 743
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
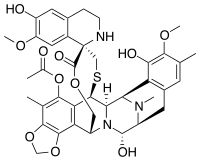
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Ecteinascidin 743 | |||||||||||||||||||||
| Molecular formula | C 39 H 43 N 3 O 11 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 761.84 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ecteinascidin 743 (also trabectedin or ET-743) is a chemotherapy drug used to treat soft tissue sarcoma and ovarian cancer . It is also being investigated in clinical trials for its suitability for the treatment of breast and prostate sarcomas.
discovery
In 1969, as part of a large-scale study of the ingredients of plants and marine life at the National Cancer Institute, it was discovered that an extract from the sea squirt Ecteinascidia turbinata had anti-carcinogenic properties. The active component ecteinascidin 743 was not characterized until 1984 by Kenneth L. Rinehart at the University of Illinois . The structure of ecteinascidin 743 is complex and has three tetrahydroisoquinoline units, 8 rings, including a ten-membered heterocyclic ring with one cysteine residue and seven chiral centers. The effect of Ecteinascidin 743 is based on binding to deoxyribonucleic acid and a disruption of the cell cycle.
synthesis
After attempts to breed the sea squirt failed and the yield was only in the ppm range, EJ Corey's working group was commissioned with the synthesis of the natural product . The synthetic route was published in 1996. A simplified synthesis was later also developed in Corey's group.
The biosynthesis of ecteinascidin 743 is believed to involve the dimerization of two tyrosine residues to form the pentacyclic core of the molecule. EJ Corey's total synthesis strategy was inspired by the proposed biosynthetic route. The synthesis uses such reactions as the Mannich reaction , the Pictet-Spengler reaction , the Curtius rearrangement and an enantioselective hydrogenation catalyzed by a chiral diphosphane- rhodium complex .
Another synthetic route is via the Ugi reaction , a one-pot multicomponent reaction which is rather unusual for the synthesis of a complex molecule.
In 2019 , Dawei Ma and co-workers presented a total synthesis that enables the synthesis of ecteinascidin 743 and the related lurbinectedin , also used as a chemotherapeutic agent, on a gram scale.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Kenneth L. Rinehart: Antitumor compounds from tunicates. In: Medicinal Research Reviews. 20, 2000, pp. 1-27, doi : 10.1002 / (SICI) 1098-1128 (200001) 20: 1 <1 :: AID-MED1> 3.0.CO; 2-A .
- ^ EJ Corey, David Y. Gin, Robert S. Kania: Enantioselective Total Synthesis of Ecteinascidin 743. In: Journal of the American Chemical Society. 118, 1996, pp. 9202-9203, doi : 10.1021 / ja962480t .
- ↑ Eduardo J. Martinez, EJ Corey: A New, More Efficient, and Effective Process for the Synthesis of a Key Pentacyclic Intermediate for Production of Ecteinascidin and Phthalascidin Antitumor Agents. In: Organic Letters. 2, 2000, pp. 993-996, doi : 10.1021 / ol0056729 .
- ↑ Atsushi Endo, Arata Yanagisawa, Masanao Abe, Shigemitsu Tohma, Toshiyuki Kan, Tohru Fukuyama: Total Synthesis of Ecteinascidin 743. In: Journal of the American Chemical Society. 124, 2002, pp. 6552-6554, doi : 10.1021 / ja026216d .
- ↑ Dawei Ma, Weiming He, Zhigao Zhang: A scalable total synthesis of Et ‐ 743 and lurbinectedin. In: Angewandte Chemie, International Edition. Accepted Article, 2019, doi : 10.1002 / anie.201900035 .