Factor V Leiden Mutation
The Leiden type factor V mutation (“ Leiden type factor five mutation”; often abbreviated as FVL mutation ) is the most common congenital thrombophilic risk marker . The risk of thrombosis in heterozygous carriers is 4-7 times higher than in the normal population. In homozygous carriers, the risk of thrombosis is likely to be about 11.5 to 26 times higher. “ Leiden ” stands for the Dutch university town where the mutation was first described in 1994. This genetic variant leads to resistance to activated protein C , i. H. Factor V cannot be inactivated adequately by activated protein C. The modified base triplet leads to the incorporation of the amino acid glutamine in place of arginine in position 506 of the protein. The resulting coagulation factor is called Factor V Leiden , or FVL for short. The change in the protein sequence creates what is known as APC resistance : normally activated factor V (FVa) is broken down by activated protein C (APC) by proteolysis and thus rendered ineffective. Due to the changed structure in the FVL, the breakdown of factor Va by APC is inhibited (it becomes “ resistant ”), and factor Va retains its coagulant effect. This leads to an imbalance of anticoagulant and anticoagulant influences, which increases the tendency to develop thromboses (thrombophilia).
Epidemiology
In Europe, around 5% of the population are heterozygous carriers of the FVL mutation. Only 0.05–0.5% are homozygous carriers who each inherited a mutated allele from father and mother (provided it is not a new mutation). The mutation occurs much less often in people of non-European descent. In evolutionary terms, the mutation offered an advantage in earlier times in the case of larger injuries, since it reduced the likelihood of bleeding to death, which is probably responsible for its frequency today. Due to natural selection , founder effects and genetic drift , the mutation can occur even more frequently in some smaller populations, for example in 12% of Swedes and Cyprus.
Historical aspects
A blood coagulation factor V deficiency was first discovered in 1955 by Max-Hermann Hörder and attributed to a blood coagulation factor V inhibitor (FVI). In 1993, the Swedish doctor Björn Dahlbäck described the factor V Leiden mutation (FVL mutation) for the first time , which ultimately causes an increased tendency towards blood clotting. As early as 1989, Dahlbäck had observed an unusual accumulation of venous thromboses in a young man , and thromboses had also occurred in other family members of the man. For a detailed examination of blood samples from the family, examination methods first had to be newly developed and refined. Finally, it was possible to detect a point mutation in the gene coding for coagulation factor V , which is located on the long arm of chromosome 1 ( gene locus 1q24.2). The mutation changes a single nucleotide at position 1691 ( adenine instead of guanine ). Dahlbäck named this genetic change, as is common among genome researchers, after the place where it was discovered - the Dutch city of Leiden - as the Factor V Leiden mutation .
Diagnosis of the FVL mutation
Indication for examination
People whose close family members - this includes grandparents, parents, siblings and their own children - have had multiple unexplained thromboses, can undergo coagulation diagnostics. This also includes testing for the FVL mutation or APC resistance . If a person has developed one or more thromboembolic disorders himself, coagulation diagnostics are also recommended. This applies all the more if there are no established risk factors (obesity, immobilization , e.g. after operations or fractures , taking hormonal contraceptives, etc.) for the occurrence of the thrombosis . In recent years, patients with repeated miscarriages (so-called habitual abortions ), stillbirths with an otherwise unclear cause and severe intrauterine growth retardation have also been found to be related to a maternal tendency to thrombosis ( thrombophilia ), so that a coagulation test of the mother and possibly a prophylaxis is appropriate.
Procedure
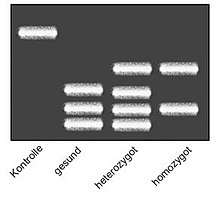
The point mutation in the factor V gene from guanine (G) to adenine (A) at position 1691 can be detected by DNA sequencing . However, since the method for direct detection is very expensive, the mutation is usually detected using a restriction fragment length polymorphism (RFLP).
To do this, the DNA of a patient's blood sample is duplicated using a polymerase chain reaction (PCR) and the gene in question is cut into nucleic acid chains of different lengths using a chemical reaction with the aid of a restriction enzyme (MnlI) . The length of the nucleic acid chains is then determined in gel electrophoresis . Restriction fragments of PCR products which carry the FVL mutation have a different size distribution than those which originate from healthy volunteers, since the mutation removes a recognition site for the restriction enzyme.
The current standard is determination by measuring the melting curve. The "real-time PCR" method is used here . A polymerase chain reaction is carried out with fluorescence-labeled nucleic acid probes which are complementary to the sequence possibly affected by the mutation. The primary dye on these probes is excited at a specific wavelength and emits light of a wavelength that excites a secondary fluorescent dye that is coupled to a second probe that is complementary to an adjacent gene sequence. Only when both probes are actually bound to the DNA are they spatially close enough for this cooperation ( called FRET ). In this way, the melting temperature of the gene probe double strand can be determined photometrically as part of a temperature gradient . If the mutation is present, the probes do not bind completely complementarily, so that the melting temperature is lowered. In the case of a heterozygous genotype, a "double" melting curve is created accordingly.
More recently, the mutation has also been discovered by chance in the context of personalized medicine and its genetic microchip examinations; the relevant single nucleotide polymorphism (SNP) bears the name rs6025.
Inheritance of the FVL mutation
The FVL mutation is inherited as an autosomal dominant trait. This means that people who inherit the FVL mutation from only one parent (heterozygous) already have a 5- to 10-fold increased risk of suffering from a thrombosis. 8% of the population of Bavaria alone have the thrombophilic risk marker (no genetic defect) in this form. If both parents pass the FVL mutation on to their child (homozygous), there is probably a 26-fold increased risk of thrombosis (maximum 7x7 = 49).
Living with the Factor V Leiden Mutation
Carriers of the Factor V Leiden mutation should avoid a thrombotic lifestyle to reduce the chance of thrombosis. These include in particular giving up smoking, avoiding obesity and, for young women, refraining from taking oral contraceptives containing estrogen . Long periods of sitting, for example on flights or buses, should be interrupted more often by moving the legs. As long as thrombosis is avoided, life expectancy is usually unaffected, and otherwise healthy heterozygotes do not require the use of anticoagulant drugs. Homozygous carriers, on the other hand, may be dependent on lifelong therapy due to the very high risk of thrombosis, especially if other risk markers are present.
During pregnancy, however, thrombosis prophylaxis with low molecular weight heparin may also be advisable in heterozygous women to prevent spontaneous abortions and blood clots, as well as after major operations that are bedridden, where rapid mobilization is particularly important.
literature
- Bjorn Dahlbäck: The discovery of activated protein c resistance . In: Journal of Thrombosis and Haemostasis . tape 1 , no. 1 , 2003, p. 3-9 .
- David H. Lee et al .: Prevalence of factor V Leiden in a Canadian blood donor population . In: Canadian Medical Association Journal . tape 155 , 1996, pp. 285 ( lac-bac.gc.ca ).
- Thrombophilia as a multigenic disease . In: Haematologica . tape 84 , no. 1 , 1999, p. 59-70 .
- Evelyn Rey et al .: Thrombophilic disorders and fetal loss: a meta-analysis . In: The Lancet . tape 361 , no. 9361 , March 15, 2003, p. 901-908 , PMID 12648968 .
References and comments
- ↑ Zotz, Sucker, Gerhardt: Importance of Thrombophilic Risk Factors for the Risk of First and Recurrence Thrombosis. (PDF) In: DRK - haemotherapy. Retrieved September 9, 2018 .
- ↑ a b c Article on rs6025 on snpedia.com, accessed January 9, 2019.
- ↑ Rogier Bertina M., Bobby PC Koeleman, Ted Koster, Frits R. Rosendaal, Richard J. Dirven: Mutation in blood coagulation factor V associated with resistance to activated protein C . In: Nature . tape 369 , no. 6475 , May 1994, ISSN 0028-0836 , pp. 64–67 , doi : 10.1038 / 369064a0 ( nature.com [accessed September 9, 2018]).
- ↑ Time bomb in the blood . In: Der Spiegel . No. 15 , 1997, pp. 222 f . ( online - April 7, 1997 ).
- ↑ Rey et al., 2003
- ↑ see picture
- ↑ see picture , columns 2, 3 and 4
- ↑ SH Neoh, MJ Brisco, FA Firgaira, KJ Trainor, DR Turner: Rapid detection of the factor V Leiden (1691 G> A) and haemochromatosis (845 G> A) mutation by fluorescence resonance energy transfer (FRET) and real time PCR . In: Journal of Clinical Pathology . tape 52 , no. 10 , October 1999, ISSN 0021-9746 , p. 766-769 , PMID 10674036 , PMC 501573 (free full text).
