Cori cycle
The Cori cycle (named after its discoverers, Gerty Cori and Carl Cori ) describes the cycle of glucose and its breakdown products between skeletal muscle and liver . The extended description includes the metabolic pathways of gluconeogenesis , glutamic acid (Glu), parts of the citric acid cycle and the urea cycle .
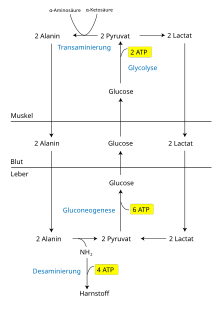
The skeletal muscle is not able to convert lactate back into glucose, even under aerobic conditions : it lacks the enzymes for gluconeogenesis . For this reason there is a circulation of metabolites between muscle and liver - the latter has the corresponding enzyme repertoire. In its original form, this organ cycle was called the “Cori cycle” . An extended form of the same, the "glucose-alanine cycle" is probably of greater importance, since it simultaneously prevents ammonia poisoning of the muscles by supplying it to the liver's detoxification apparatus (the urea cycle ).
Cori cycle
When muscles are exercised, a certain lack of oxygen quickly develops in the muscle. Under these more anaerobic conditions, the respiratory chain in the mitochondrion slows down and energy is mainly generated by glycolysis. On the other hand, pyruvate is less degraded over the citric acid cycle. Instead, pyruvate reacts anaerobically to form lactic acid . NAD + is regenerated for glycolysis. Lactic acid is released into the bloodstream as lactate - this path is shown in abbreviated form in the figure (see pyruvate ). The liver absorbs lactate from the blood and converts it back into glucose via gluconeogenesis via oxaloacetate . This glucose can - depending on the current status of the energy supply - be supplied to the liver's energy store as glycogen or released into the bloodstream in order to supply the muscles again.
However, it should be noted that this is not a closed energy cycle. More energy has to be expended in gluconeogenesis in the liver than is produced in glycolysis in the muscle. This is because in gluconeogenesis the strongly endothermic reaction of pyruvate to phosphoenolpyruvate (PEP) is bypassed in an energy-intensive manner , while the energy released in the exothermic reaction of fructose-1,6-BP to fructose-6-P or glucose-6 -P to glucose is not used. In gluconeogenesis from pyruvate, 4 ATP , 2 GTP and 2 NADH must be used for each molecule of glucose . In glycolysis, however, only 2 ATP and 2 NADH are produced from one molecule of glucose.
Glucose-Alanine Cycle
Proteins are broken down into amino acids in the cytosol . These in turn are deaminated by transamination and the remaining carbon structure is introduced into the citric acid cycle . The amino group of the amino acids is temporarily transferred to the cofactor pyridoxal phosphate (PLP) during transamination ; PLP becomes pyridoxamine phosphate (PAMP). The alanine aminotransferase (ALAT, ALT) (also called glutamate pyruvate transaminase, GPT) transfers the amino grouping from PAMP to pyruvate in the muscle. This creates alanine and regenerated PLP, which can then take up new amino groups. Alanine is transported via the blood to the liver, where ALAT turns PLP and alanine into PAMP and pyruvate, which can be used for gluconeogenesis and is sent back to the extrahepatic cells as glucose.
ALAT transfers the amino group from PAMP to α-ketoglutarate. The resulting glutamate is converted in the mitochondria of the liver cell by glutamate dehydrogenase (GLDH) to α-ketoglutarate and NH 3 ; the latter is converted by carbamoyl phosphate synthetase I with CO 2 to carbamoyl phosphate , which flows into the urea cycle . The second NH 2 group of urea is supplied by a transamination product of aspartate (Asp), which in turn is split into arginine and fumarate . Urea is finally split off from the arginine . Fumarate can be regenerated to aspartate via malate and oxaloacetate (aspartate cycle). Urea is excreted through the kidneys .
In contrast to the Cori cycle, the alanine cycle not only regenerates carbohydrates, but also removes NH 3 from the muscle. For this, however, energy must also be expended in the urea synthesis of the liver in order to dispose of NH 3 .
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6 edition, Spektrum Akademischer Verlag, Heidelberg 2007. ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 3rd edition, John Wiley & Sons, New York 2004. ISBN 0-471-19350-X .