House bases
In Hauser bases , also known as magnesium amide referred, is magnesium compounds used in the organic chemistry as bases for metalation be used, and for the first time in 1947 by Charles R. Hauser have been described. Compared to organolithium compounds , these compounds have kovalentere and thus less reactive metal - ligand - bonds . As a consequence, this leads to a higher tolerance towards functional groups and a significantly higher oneChemoselectivity . Hauserbases can also be used for reactions at room temperature, whereas reactions with organolithium compounds tend to be carried out at low temperatures, usually −78 ° C.
structure
Solid structure
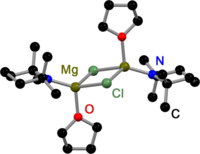
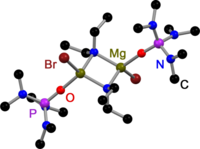
Like all Grignard dimers , the house bases derived from 2,2,6,6-tetramethylpiperidine (TMP) and hexamethyldisilazane (HMDS) are bridged in the solid state by the halogens. In contrast to Gringnard compounds, there are also amido-bridged house bases. All have in common that they are bridged by sterically less demanding amido ligands such as Et 2 N− , Ph 3 P = N− or i Pr 2 N− . The replacement of the halogen bridges is probably related to the steric demands of the amide ligands.
| . | . | . |
Structure in solution
Although much is known about the possibilities of using house bases, little is known about their behavior in solution. One reason for this lies in the complex behavior that they exhibit in solution. One suggestion was that there are similarities to the Schlenk equilibrium of the Gringnard reagents in which more than one magnesium-containing species exists. In 2016, Neufeld et al. demonstrate such a Schlenk-like behavior by means of DOSY-NMR experiments :
The equilibrium shows a high temperature dependence. At high temperatures, the heteroleptic species is mainly present, while at low temperatures the homoleptic species is present. In addition, the THF solution contains dimeric species that are bridged by chlorides and amides , although alkyl magnesium chlorides do not dimerize there. At low temperatures and an excess of magnesium chloride , MgCl 2 co-coordinating species are also found in the solution .
use
In general, house bases, like organolithium compounds or metal amides, are used as metalating reagents. The breakthrough of synthesis instructions with Hauserbases took place in the 1980s and 1990s. Eaton and his co-workers were able to show that i Pr 2 NMgBr selectively forms magnesiate carboxamides in orthoposition .
Later, Kondo , Sakamo, and their co-workers showed that i Pr 2 NMgX (X = Cl, Br) serves as a selective deprotonation reagent (only at the 2-position) for heterocyclic thiophenes and phenylsulphonyl-substituted indoles .
A major disadvantage of house bases is their poor solubility in THF. As a consequence, the metalation rate is slow and a large excess of base is required (usually 10 equivalents). This fact complicates the functionalization of the metalated intermediate as an electrophile . The solubility and thus also the reactivity can, however, be achieved by adding stoichiometric amounts of lithium chloride . These so-called Turbo Hauserbases such as TMPMgCl·LiCl and i Pr 2 NMgCl·LiCl are partly commercially available and show an increased kinetic basicity, excellent regioselectivity and high tolerance towards functional groups in many aromatic and heteroaromatic substrates.
presentation
Hauserbases can be prepared by mixing an amine with a Grignard reagent (X = Cl, Br, I):
Commercially available home bases
i Pr 2 NMgX
TMPMgX (TMP = 2,2,6,6, tetramethylpiperidino )
X = Cl, Br
Individual evidence
- ^ Charles R. Hauser, Howard G. Walker: Condensation of Certain Esters by Means of Diethylaminomagnesium Bromide 1,2 . In: Journal of the American Chemical Society . tape 69 , no. 2 , February 1947, ISSN 0002-7863 , p. 295-297 , doi : 10.1021 / ja01194a040 .
- ^ Robert Li-Yuan Bao, Rong Zhao, Lei Shi: Progress and developments in the turbo Grignard reagent i-PrMgCl LiCl: a ten-year journey . In: Chemical Communications . tape 51 , no. 32 , 2015, ISSN 1359-7345 , p. 6884-6900 , doi : 10.1039 / C4CC10194D .
- ↑ Ömer Seven, Michael Bolte, Hans-Wolfram Lerner: Di-μ-bromido-bis [(diethyl ether-κ O) (2,4,6-trimethylphenyl) magnesium]: the mesityl Grignard reagent . In: Acta Crystallographica Section E Structure Reports Online . tape 69 , no. 7 , July 15, 2013, ISSN 1600-5368 , p. m424 – m424 , doi : 10.1107 / S1600536813017108 , PMID 24046588 , PMC 3772445 (free full text).
- ^ Pablo García-Álvarez, David V. Graham, Eva Hevia, Alan R. Kennedy, Jan Klett: Unmasking Representative Structures of TMP-Active Hauser and Turbo-Hauser Bases . In: Angewandte Chemie International Edition . tape 47 , no. 42 , October 6, 2008, p. 8079-8081 , doi : 10.1002 / anie.200802618 .
- ↑ Kuo-Ching Yang, Chung-Cheng Chang, Jyh-Yuan Huang, Chih-Chien Lin, Gene-Hsiang Lee: Synthesis, characterization and crystal structures of alkyl-, alkynyl-, alkoxo- and halo-magnesium amides . In: Journal of Organometallic Chemistry . tape 648 , no. 1-2 , April 2002, pp. 176–187 , doi : 10.1016 / S0022-328X (01) 01468-1 ( elsevier.com [accessed October 26, 2019]).
- ^ Pablo García-Álvarez, David V. Graham, Eva Hevia, Alan R. Kennedy, Jan Klett: Unmasking Representative Structures of TMP-Active Hauser and Turbo-Hauser Bases . In: Angewandte Chemie International Edition . tape 47 , no. 42 , October 6, 2008, p. 8079-8081 , doi : 10.1002 / anie.200802618 .
- ↑ Andrei S. Batsanov, Philip D. Bolton, Royston CB Copley, Matthew G. Davidson, Judith AK Howard: The metallation of imino (triphenyl) phosphoranes by ethyl magnesium chloride: The synthesis, isolation and X-ray structure of [Ph 3 P = NMgCl · O = P (NMe2) 3] 2 . In: Journal of Organometallic Chemistry . tape 550 , no. 1-2 , January 1998, pp. 445-448 , doi : 10.1016 / S0022-328X (97) 00550-0 .
- ↑ David R. Armstrong, Pablo García-Álvarez, Alan R. Kennedy, Robert E. Mulvey, John A. Parkinson: Diisopropylamide and TMP Turbo-Grignard Reagents: A Structural Rationale for their Contrasting Reactivities . In: Angewandte Chemie International Edition . tape 49 , no. 18 , April 19, 2010, pp. 3185-3188 , doi : 10.1002 / anie.201000539 .
- ↑ Neufeld, R .: DOSY External Calibration Curve Molecular Weight Determination as a Valuable Methodology in Characterizing Reactive Intermediates in Solution. In: eDiss, Georg-August-Universität Göttingen. 2016.
- ↑ Roman Neufeld, Dietmar Stalke: Accurate molecular weight determination of small molecules via DOSY NMR by using external calibration curves with normalized diffusion Coefficients . In: Chemical Science . tape 6 , no. 6 , 2015, ISSN 2041-6520 , p. 3354–3364 , doi : 10.1039 / C5SC00670H , PMID 29142693 , PMC 5656982 (free full text).
- ^ Roman Neufeld, Thorsten L. Teuteberg, Regine Herbst-Irmer, Ricardo A. Mata, Dietmar Stalke: Solution Structures of Hauser Base i Pr 2 NMgCl and Turbo-Hauser Base i Pr 2 NMgCl·LiCl in THF and the Influence of LiCl on the Schlenk Equilibrium . In: Journal of the American Chemical Society . tape 138 , no. 14 , April 13, 2016, ISSN 0002-7863 , p. 4796-4806 , doi : 10.1021 / jacs.6b00345 .
- ^ Roman Neufeld, Thorsten L. Teuteberg, Regine Herbst-Irmer, Ricardo A. Mata, Dietmar Stalke: Solution Structures of Hauser Base i Pr 2 NMgCl and Turbo-Hauser Base i Pr 2 NMgCl·LiCl in THF and the Influence of LiCl on the Schlenk Equilibrium . In: Journal of the American Chemical Society . tape 138 , no. 14 , April 13, 2016, ISSN 0002-7863 , p. 4796-4806 , doi : 10.1021 / jacs.6b00345 .
- ↑ Philip E. Eaton, Chih Hung Lee, Yusheng Xiong: Magnesium amide bases and amido-Grignards. 1. Ortho magnesiation . In: Journal of the American Chemical Society . tape 111 , no. September 20 , 1989, ISSN 0002-7863 , pp. 8016-8018 , doi : 10.1021 / ja00202a054 .
- ↑ Manabu Shilai, Yoshinori Kondo, Takao Sakamoto: Selective metallation of thiophene and thiazole rings with magnesium amide base . In: Journal of the Chemical Society , Perkin Transactions 1 . No. 4 , 2001, p. 442-444 , doi : 10.1039 / b007376h .
- ↑ Yoshinori Kondo, Akihiro Yoshida, Takao Sakamoto: Magnesiation of indoles with magnesium amide bases . In: Journal of the Chemical Society, Perkin Transactions 1 . No. 19 , 1996, ISSN 0300-922X , p. 2331 , doi : 10.1039 / p19960002331 .
- ↑ New Reagents for Selective Metalation, deprotonation, and Additions. Retrieved October 26, 2019 .
- ↑ David Tilly, Floris Chevallier, Florence Mongin, Philippe C. Gros: Bimetallic Combinations for Dehalogenative Metalation Involving Organic Compounds . In: Chemical Reviews . tape 114 , no. 2 , January 22, 2014, ISSN 0009-2665 , p. 1207-1257 , doi : 10.1021 / cr400367p .
- ↑ Thomas Klatt, John T. Markiewicz, Christoph Sämann, Paul Knochel: Strategies To Prepare and Use Functionalized Organometallic Reagents . In: The Journal of Organic Chemistry . tape 79 , no. 10 , May 16, 2014, ISSN 0022-3263 , p. 4253-4269 , doi : 10.1021 / jo500297r .