Hydramethylnon
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
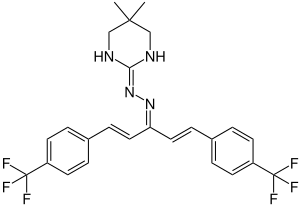
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hydramethylnon | |||||||||||||||
| other names |
1,5-bis [4- (trifluoromethyl) phenyl] -1,4-pentadien-3-one 2- (1,4,5,6-tetrahydro-5,5-dimethyl-2-pyrimidinyl) hydrazone |
|||||||||||||||
| Molecular formula | C 25 H 24 F 6 N 4 | |||||||||||||||
| Brief description |
odorless, yellow to yellow-brown solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 494.48 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.23 g cm −3 |
|||||||||||||||
| Melting point |
187.5 ° C |
|||||||||||||||
| Vapor pressure |
<0.00001 hPa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hydramethylnon is an active ingredient for crop protection and a chemical compound from the group of trifluoromethylaminohydrazones .
Extraction and presentation
Hydramethylnon can be obtained by a condensation reaction of 4-trifluoromethylbenzaldehyde with acetone and the further reaction of the intermediate product formed with a hydrazine derivative .
properties
As a technical product, hydramethylnon is a yellow to yellow-brown crystalline solid with a characteristic odor of vegetable oil. When exposed to light, it is rapidly broken down by photolysis .
use
Hydramethylnon is used as an insecticide . It was originally developed by American Cyanamid in 1977 and is commercially available under the names Amdro, Combat, Faslan, Maxforce, Sensible and Siege, for example. These products are slow-acting poisons that are mainly used against ants on grassy areas, pastureland, ornamental and sports lawns and similar non-agricultural areas. In the USA, hydramethylnon is also used in households and in commercial operations (outside the food industry) against species of ants, silverfish , termites and cockroaches . The action of hydramethylnon is based on the inhibition of the production of ATP in the mitochondria .
Admission
In the EU countries including Germany and Austria as well as in Switzerland no pesticides with the active ingredient hydramethylnon are permitted.
Individual evidence
- ↑ extoxnet: hydramethylnon
- ↑ a b c d e f Datasheet Hydramethylnon at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ a b c d EPA: Reregistration Eligibility Decision (RED) Hydramethylnon (PDF file; 426 kB).
- ↑ a b Entry on 5,5-dimethyl-perhydro-pyrimidin-2-one-alpha- (4-trifluoro-methylstyryl) -alpha- (4-trifluoromethyl) cinnamylidene hydrazone in the GESTIS substance database of the IFA , accessed on January 8th 2020(JavaScript required) .
- ↑ Entry on 5,5-dimethyl-perhydro-pyrimidin-2-one-α- (4-trifluoromethylstyryl) -α- (4-trifluoromethyl) cinnamylidenehydrazone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 12 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Bruce E. Smart, JC Tatlow: Organofluorine chemistry: principles and commercial applications . Springer US, 1994, ISBN 978-0-306-44610-8 ( page 250 in the Google book search).
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 ( page 1098 in the Google book search).
- ^ Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals . Royal Soc of Chemistry, 1999, ISBN 978-0-85404-499-3 ( page 760 in the Google book search).
- ↑ Mode of action of the most important biocides, part 1 (PDF file; 996 kB).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on hydramethylnon in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 8, 2016.


