Imibenconazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
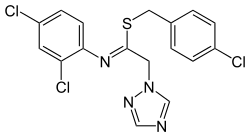
|
||||||||||||||||
| ( Z ) isomer | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Imibenconazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 13 Cl 3 N 4 S | |||||||||||||||
| Brief description |
white crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 411.74 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
89.5-90 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Imibenconazole is a mixture of two cis , trans isomeric chemical compounds from the group of triazoles and a fungicide introduced by Hokko Chemical Industry in the late 1980s .
Extraction and presentation
Imibenconazole can be prepared starting from 1,2-dichloroethane- N -2,4-dichlorophenylimide, 1,2,4-triazole and 4-chlorobenzyl mercaptan .
use
Imibenconazole is a systemic fungicide that has both protective and curative effects. The active ingredient is one of the azole fungicides , which inhibit C14 demethylase in sterol biosynthesis.
Imibenconazole is used to combat anthracnose , scab and rust fungi in pome fruit and citrus cultivation.
Admission
No plant protection products containing this active ingredient are permitted in the EU or Switzerland .
Individual evidence
- ↑ a b c d Entry on imibenconazole. In: Römpp Online . Georg Thieme Verlag, accessed on November 9, 2014.
- ↑ a b Crop Protection Handbook 2014 . 100th edition. MeisterPro, Willoughby, Ohio 2014, pp. 358 .
- ↑ a b Data sheet Imibenconazole solution, 100 ng / μL in acetonitrile from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 708 ( limited preview in Google Book search).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Imibenconazole in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.

